Protease Stabilized, Pegylated Insulin Analogues
a technology of pegylated insulin and protease, which is applied in the direction of peptide/protein ingredients, drug compositions, metabolic disorders, etc., can solve the problems that the administration of therapeutic peptides or proteins is often limited to parenteral routes rather than the preferred oral administration, and achieves high potential, high degree of bioavailability, and high apparent potency and/or bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure (A)
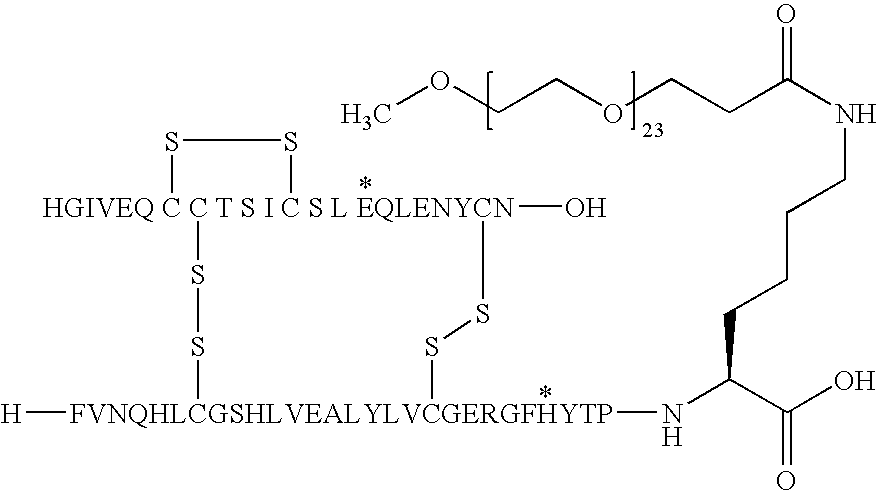
[0278]A14E, B25H, B29K(M-3-mdPEG24-Propionyl), desB30 Human Insulin
[0279]A14E, B25H desB30 human insulin (1.5 g) was dissolved in 0.1 M Na2CO3 (34 ml) and pH was adjusted to 10 with 1N NaOH. mdPEG24-SPA (0.45 g, Quanta BioDesign Ltd.) dissolved in MeCN (16.8 ml) was added and the mixture was slowly stirred for 1 hour. Water (25 ml) was added, pH was adjusted to 5.5 with 1N HCl and the mixture was lyophilised. The title compound was obtained by preparative HPLC purification. Column: C18, 3 cm. A-Buffer: 0.1% TFA in MiliQ Water; B-buffer: 0.1% TFA in acetonitrile. Gradient 10-55% B over 45 min. Yield: 650 mg.
[0280]MALDI-MS (matrix: HCCA); m / z: 6762, calcd: 6762.
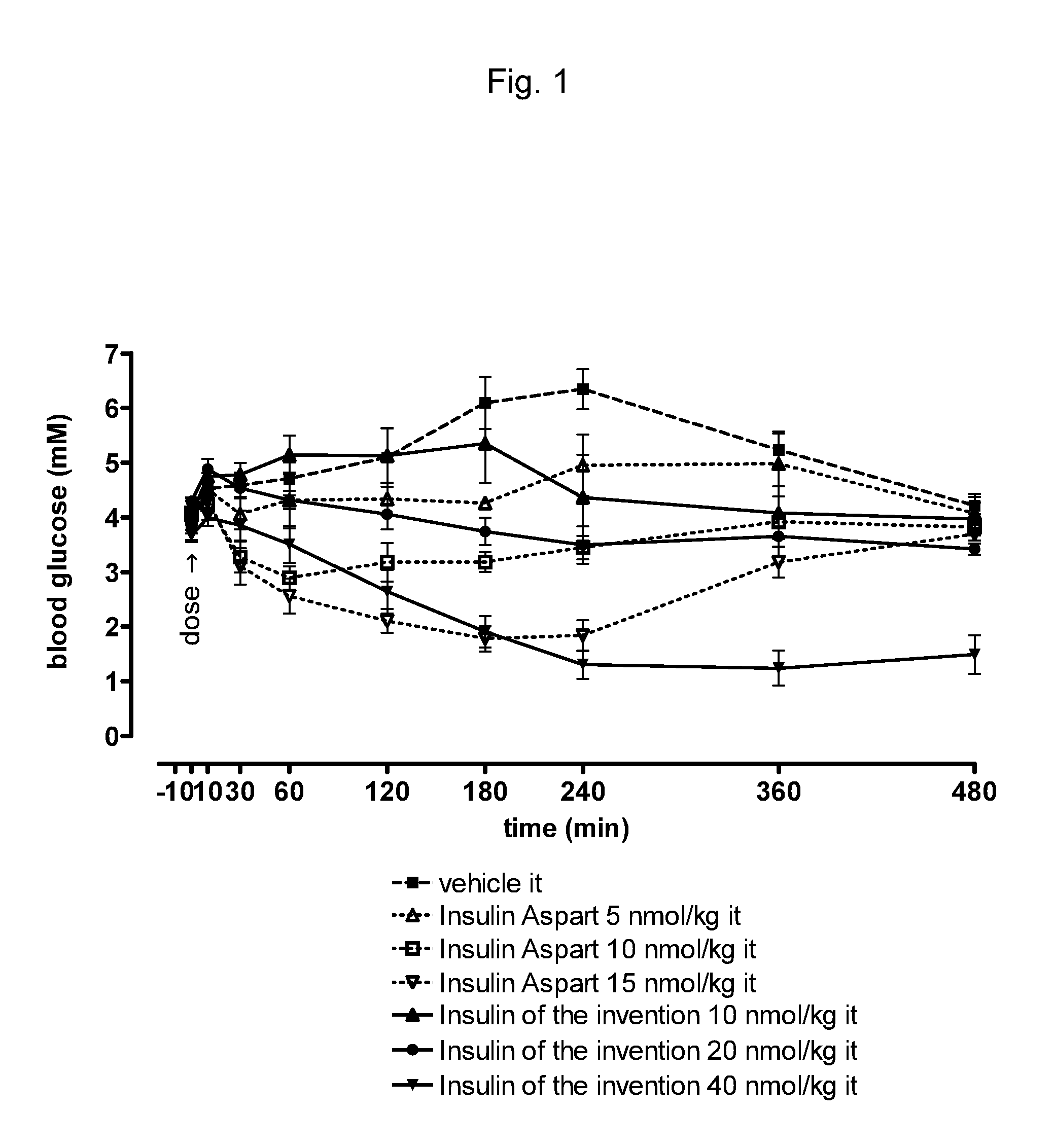
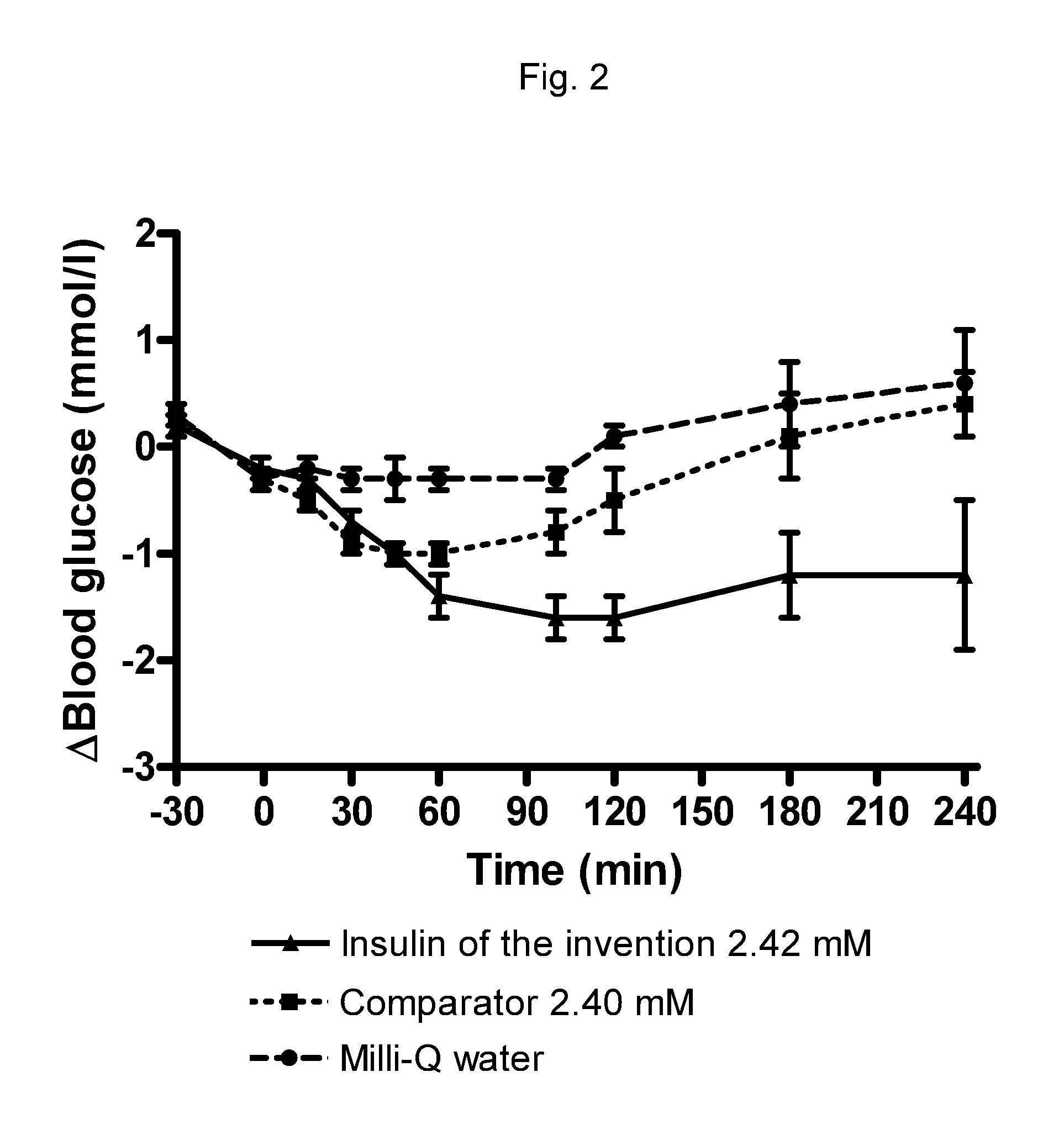
[0281]This compound has substantial protracted pulmonary efficacy.
[0282]The compound of example 1 (i.e. A14E, B25H, B29K(Nε-3-mdPEG24-propionyl), desB30 human insulin) and insulin aspart were tested by rat intratracheal drop instillation by the procedure described in example 10 below. The number of animals ...
example 2
General Procedure (A)
[0283]A14E, B25H, B29K(M-3-mPEG2.000-Propionyl), desB30 Human Insulin
[0284]MALDI-MS (matrix: sinapinic acid); m / z: 7850 (broad).
example 3
General Procedure (A)
[0285]A14E, B25H, B29K(Ar3-{mPEG750}Propionylcarbamoyl), desB30 Human Insulin
[0286]MALDI-MS (matrix: sinapinic acid); m / z: 6570 (broad).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Hydrophobicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com