Conjugates of neurotensin or neurotensin analogs and uses thereof
a neurotensin and analog technology, applied in the field of conjugates of neurotensin or neurotensin analogs and uses thereof, can solve the problems of delayed onset of hypothermia, difficulty in sustained maintenance of hypothermia, complex and expensive methods, etc., and achieve the effect of reducing the frequency or severity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of a neurotensin-Angiopep-2 conjugate
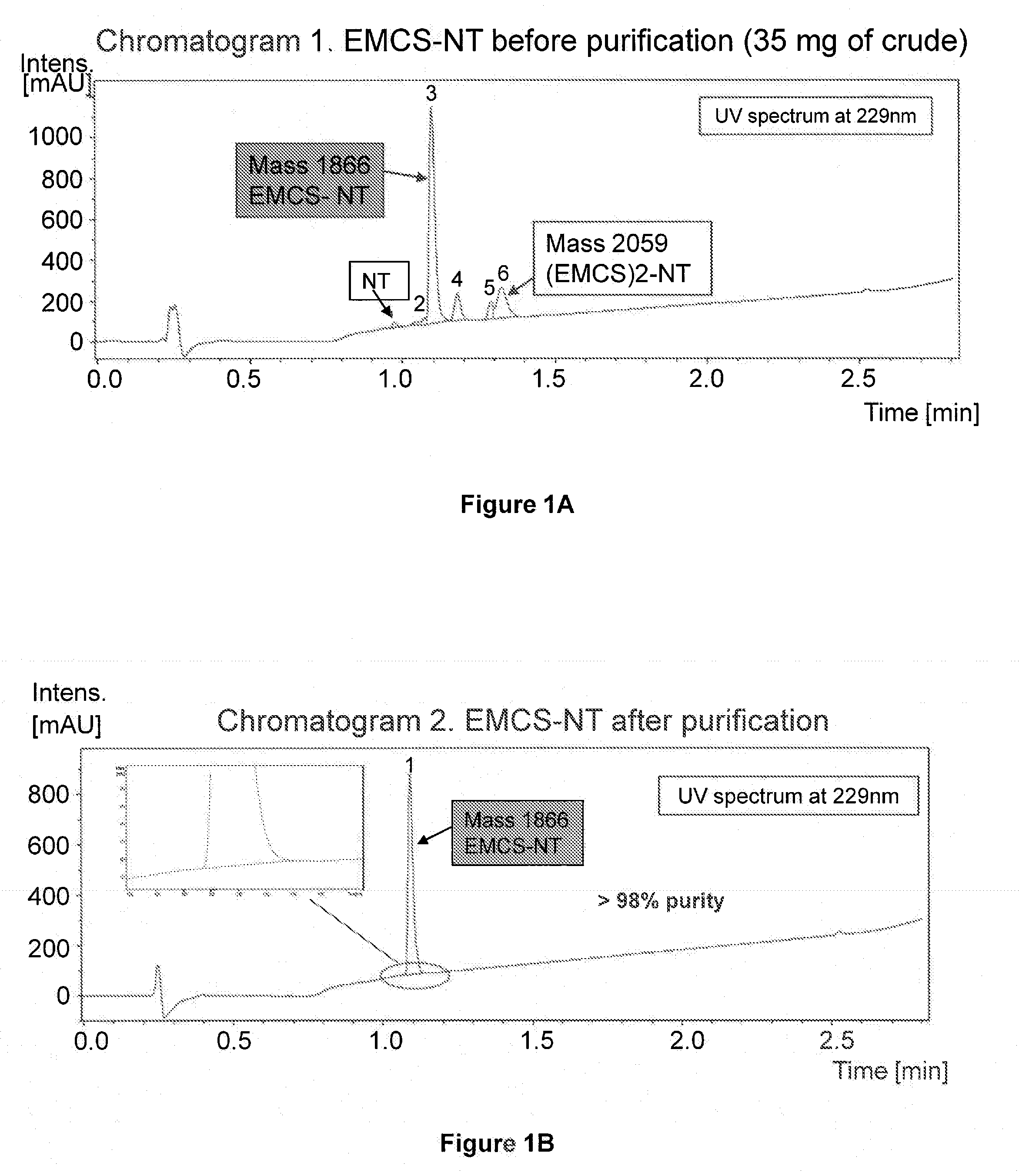
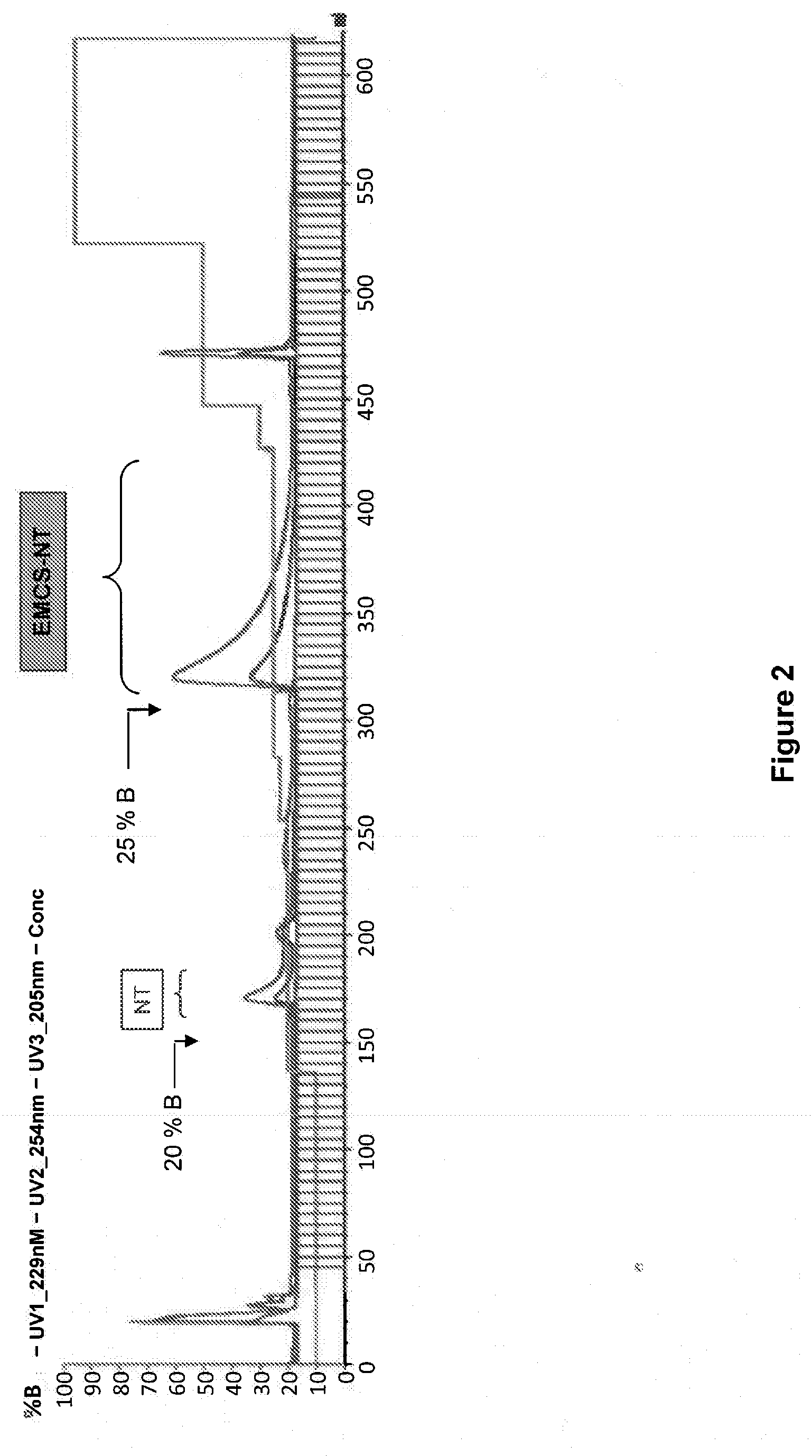
[0145]An exemplary neurotensin-Angiopep-2 conjugate was synthesized using the scheme described below. As used in these examples, the abbreviation NT refers to the pE-substituted neurotensin peptide described below.
[0146]Neurotensin Peptide Synthesis
[0147]pELYENKPRRPYIL-OH, where the unusual amino acid L-pyroglutamic acid (pE) is used, was synthesized using SPPS (Solid phase peptide synthesis). SPPS was carried out on a Protein Technologies, Inc. Symphony® peptide synthesizer using Fmoc (9-fluorenylmethyloxycarbonyl) amino-terminus protection. The peptide was synthesized on a 100 μmol scale using a 5-fold excess of Fmoc-amino acids (200 mM) relative to the resin. Coupling was performed by a pre-loaded Fmoc-Leu-Wang resin (0.48 mmol / g) for carboxyl-terminus acids using 1:1:2 amino acid / activator / NMM in DMF with HBTU (2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) and NMM (N-methylmorpholine). Deprotection was car...
example 2
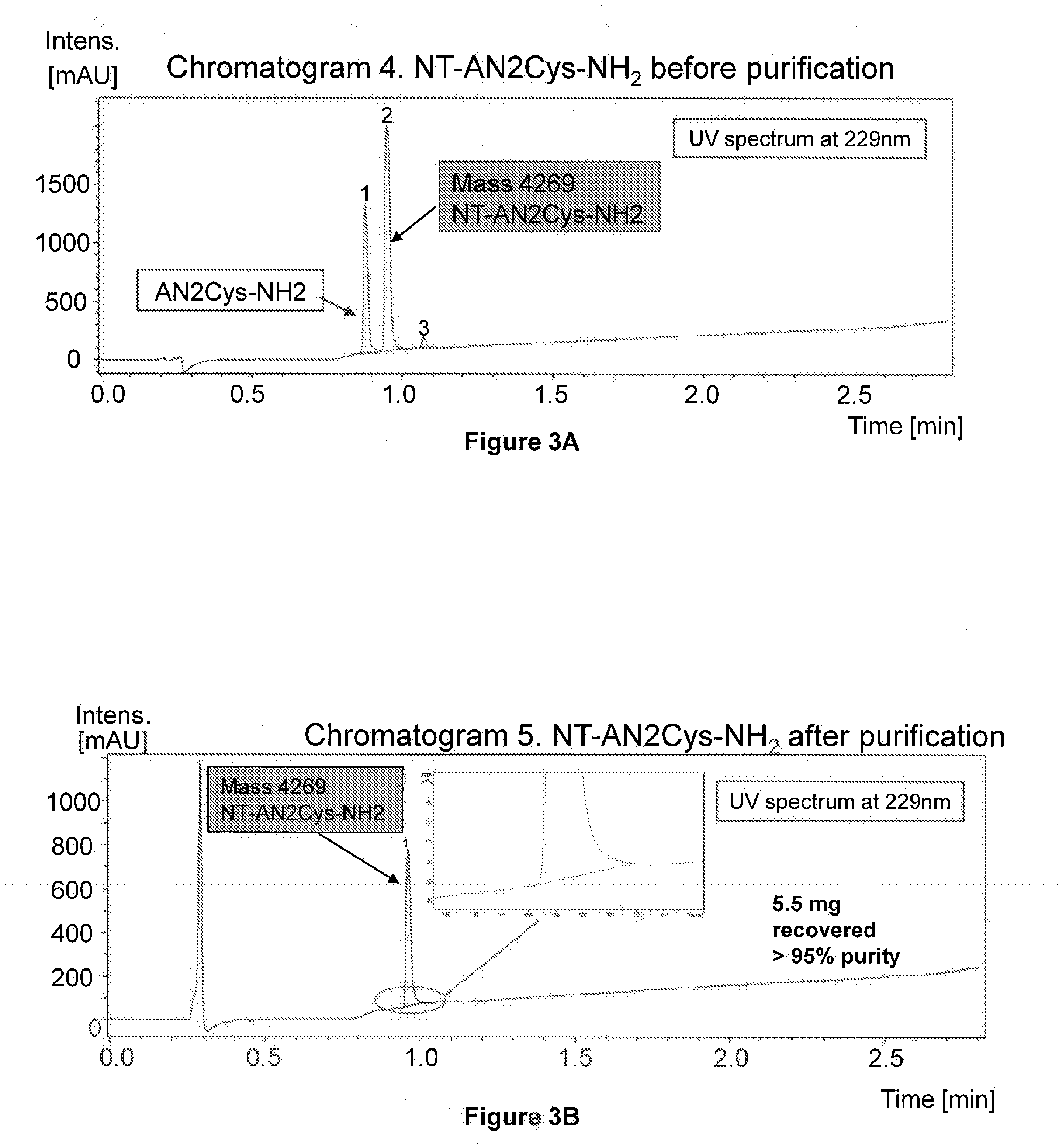
Characterization of the NT-AN2Cys-NH2 conjugate
[0159]To investigate the pharmacological efficacy and brain penetration of the NT-AN2Cys-NH2 (NT-An2) conjugate, we monitored its effect on the body temperature of mice (FIG. 5). The temperature of mice was unaffected by intravenous administration of 1 mg / kg NT or the saline control. By contrast, intravenous administration of an equivalent dose of the conjugate (2.5 mg / kg) resulted in a rapid decrease in the body temperature, leading to hypothermia. The injection of a higher dose (5 mg / kg) of NT-An2 caused a stronger decrease in body temperature indicating that the effect of NT-An2 is dose dependent.
[0160]We also tested whether higher doses of the conjugate would results in greater induction of hypothermia. Mice were administered 5, 15, or 20 mg / kg of the conjugate, and the reduction in body temperature following administration was monitored for 120 minutes following administration. Small differences between these higher doses were obse...
example 3
Induction of Sustained Hypothermia Using Angiopep-NT Conjugates
[0163]We performed an additional experiments to test whether the conjugates were able to induce sustained hypothermia in mice and rats.
[0164]In a first experiment, mice first received an intravenous 5 mg / kg bolus injection of NT-An2, followed by an intravenous infusion (10 mg / kg / hr) 1 hour later for a duration of 2.5 hours. The body temperature continued to decrease during the infusion, reaching a nadir of −11° C. (FIG. 10). After the end of the infusion, body temperature slowly returned to 37° C., and the animals recovered.
[0165]A similar experiment was performed in rats. Here the rats were administered an intravenous bolus injection of 20 mg / kg NT-An2 immediately followed by a 20 mg / kg / hr infusion for 3.5 hours. This resulted in a maximal temperature drop of about 3.5° C. after 90 minutes (FIG. 11).
[0166]Sustained hypothermia experiments were performed using a intravenous bolus injection of 20 mg / kg of NT-An2 immediate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com