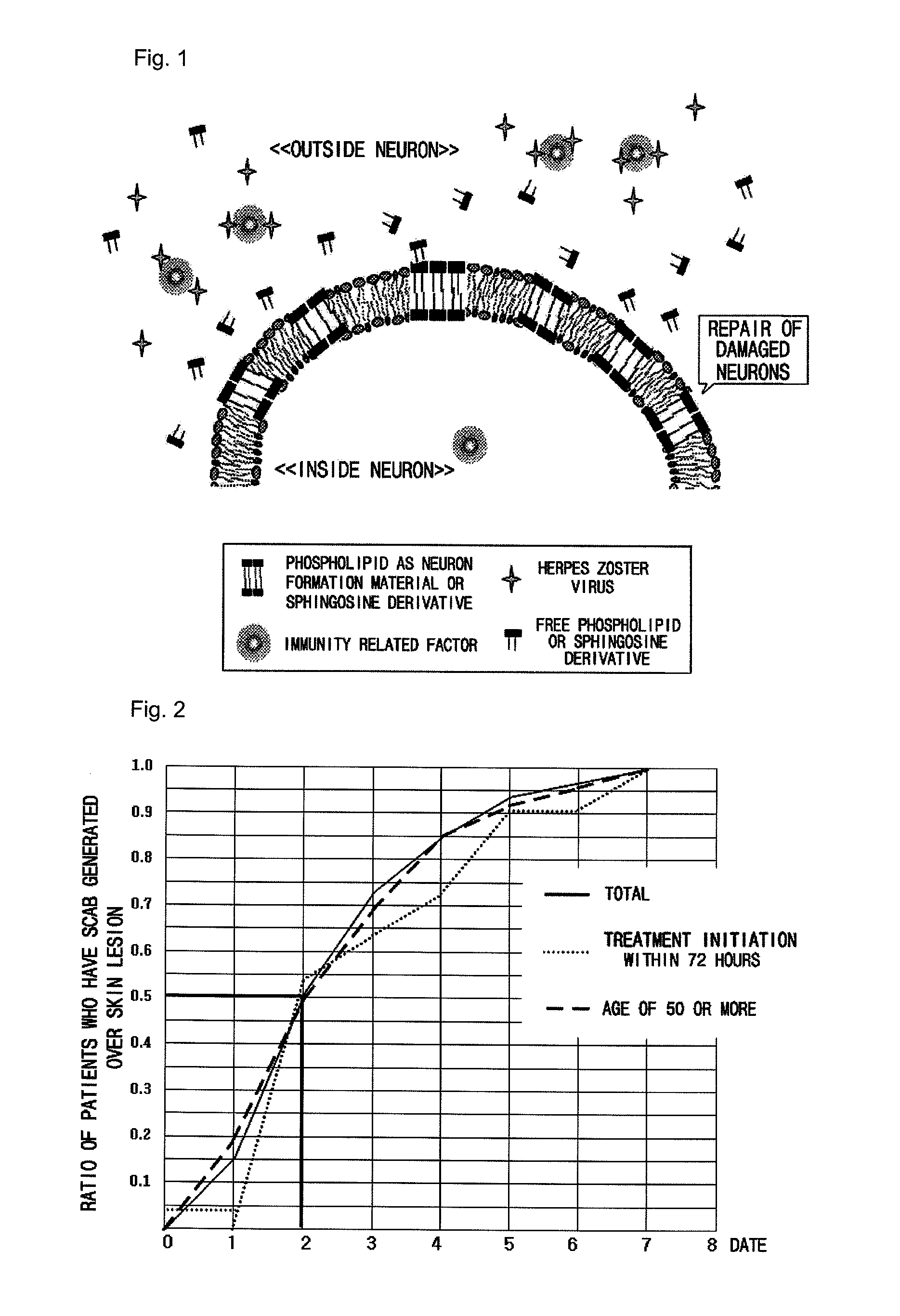
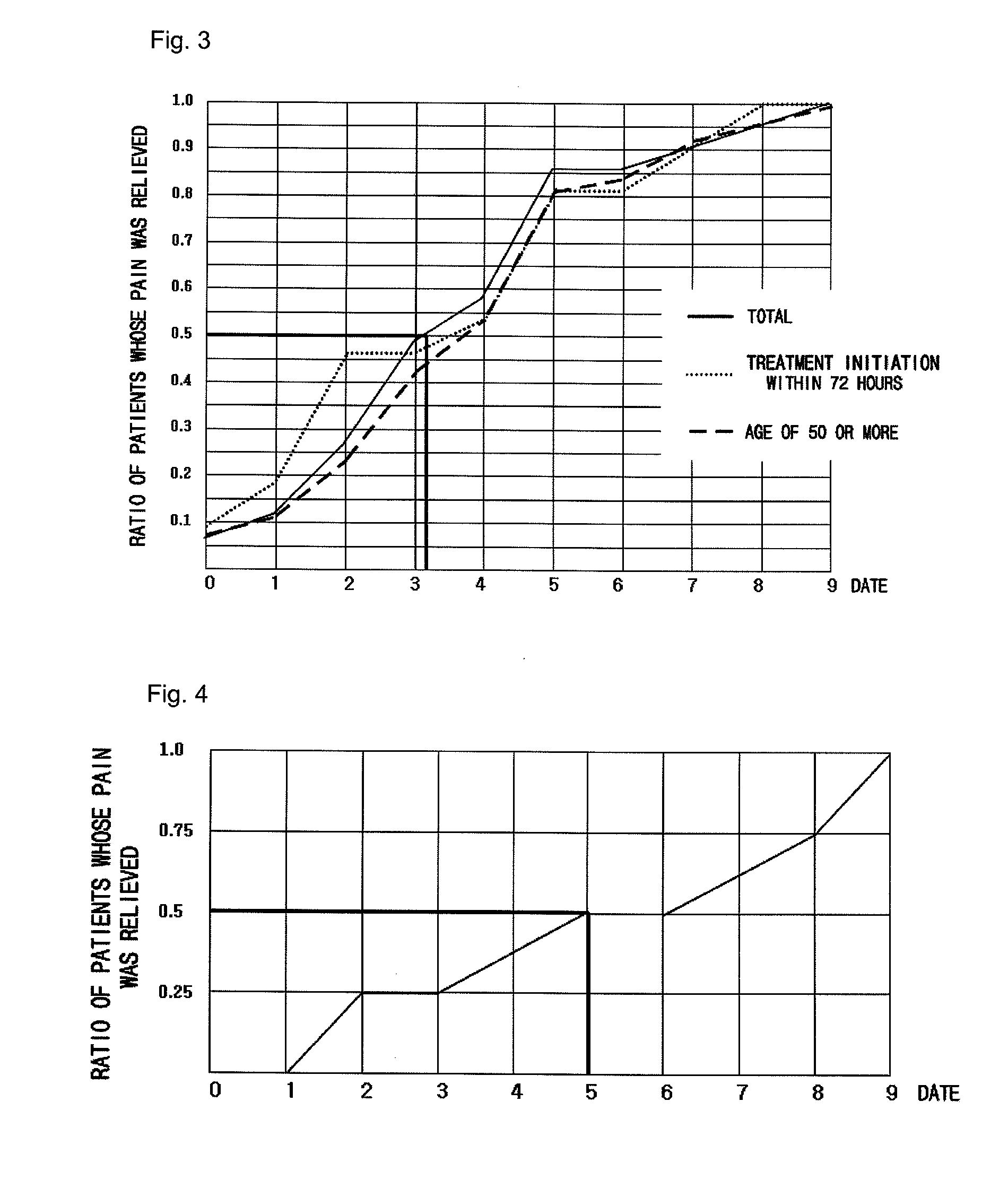
Pharmaceutical composition for preventing or treating neuronal damage and neurological diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of H-Z Cream
[0052]0.7 g of potassium hydroxide and 10 mL of glycerin were dissolved in 75 mL of purified water in a 200 mL beaker and then the solution was heated at a temperature of 80 to 85° C. This solution was added to a liquid stearic acid prepared by completely melting 15 g of stearic acid in a 250 mL beaker in a water bath at a temperature of 80 to 85° C. and stirred and cooled to room temperature, thereby preparing a vanishing cream.
[0053]100.7 g of the prepared vanishing cream was added to 229.0 g of aloe vera gel and the mixture was sufficiently mixed. Then, 16.03 g of Oronia Soybean Lecithin (supplier: Deraco Natural Source Company LTD.) as a phospholipid or sphingosine derivative, 1.60 g of alpha-tocopherol, and 0.50 g of propyl benzoate were each added thereto in small portions while stirring, thereby preparing a light yellowish cream. The resultant composition included 1.7 wt % of phospholipid or a sphingosine derivative.
example 2
Preparation of H-Z Cream
[0054]4.00 g of Tween #80 was added to 200.0 g of aloe vera gel and the mixture was sufficiently mixed. Then, 20.00 g of Oronia Soybean Lecithin (supplier: Deraco Natural Source Company LTD.), 2.00 g of alpha-tocopherol, and 0.30 g of propyl benzoate were each added thereto in small portions while stirring, thereby preparing a light yellowish cream. The resultant composition included 3.24 wt % of phospholipid or a sphingosine derivative.
example 3
Preparation of H-Z Cream
[0055]14.00 g of Tween #80 was added to 127.0 g of aloe vera gel and the mixture was sufficiently mixed. Then, 70.00 g of GL-90E (supplier: GoshenBiotech) as phospholipid or a sphingosine derivative, 7.00 g of alpha-tocopherol, and 0.30 g of propyl benzoate were each added thereto in small portions while stirring, thereby preparing a light yellowish cream. The resultant composition included 29.1 wt % phospholipid or a sphingosine derivative.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com