Compositions and methods for mammalian genetics and uses thereof
a technology of mammalian genetics and composition methods, applied in the field of mammalian genetic composition and methods, can solve the problems of limiting the contribution of mutagenesis-based genetics to the understanding of human diseases, generating and recovering,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Characterization and Retroviral Infection of KBM7 Subclones
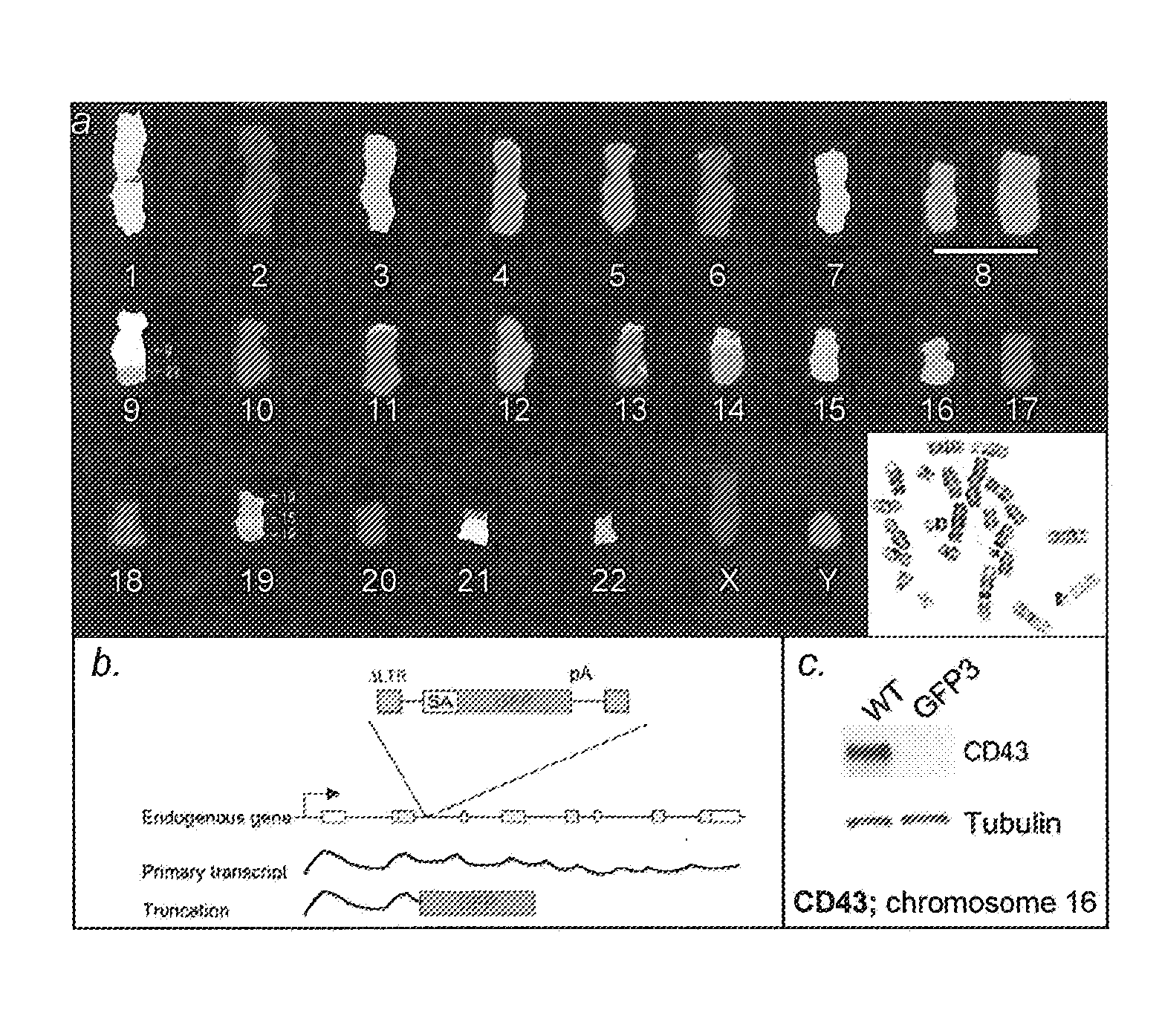
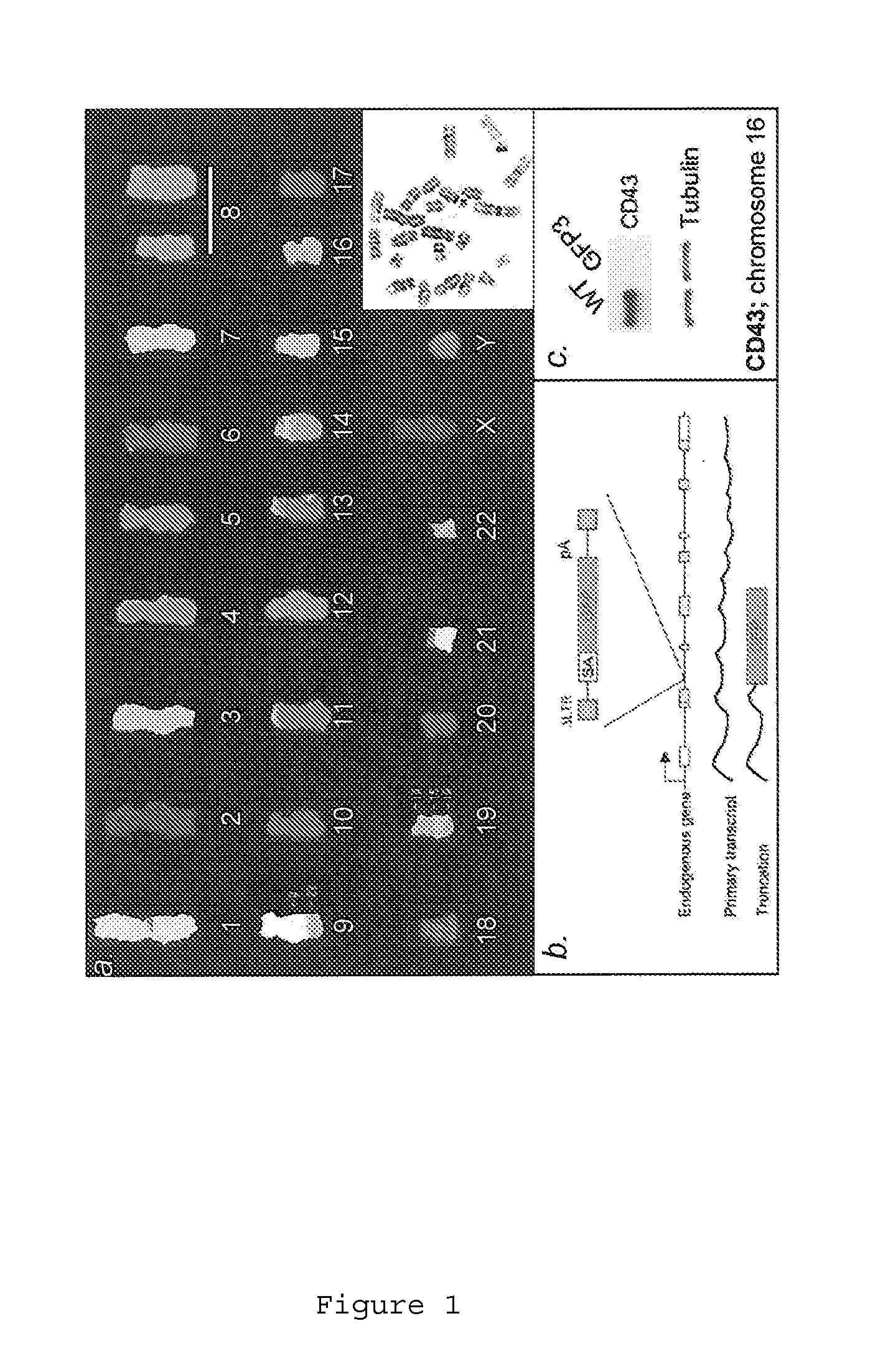
[0149]We first characterized a haploid genome setting in human cells that we believed would be permissive for efficient forward genetic approaches. A subclone of the CML cell line KBM7 has been described to carry a near haploid chromosome set [14]. First we examined if this cell line (generously provided by Dr. B. H. Cochran, Tufts University School of Medicine, Boston, Mass.) could be easily propagated, was tolerant to viral infection and could be efficiently subcloned. The term “KBM7 cell line” is used herein to refer to this near-haploid cell line or to a subclone thereof. Cells of the KBM7 cell line of a subclone thereof may be referred to as “KBM7 cells”. KBM7 cells had a high subcloning efficiency (of around ˜80%), and several of the subclones were examined further. The KBM7 subclones proliferated readily with a generation time of approximately 24 hrs and could be maintained at sparse and very high cell densities (e.g....
example 2
Selection of Gene Trap Vectors for Insertional Mutagenesis in KBM7 Cells
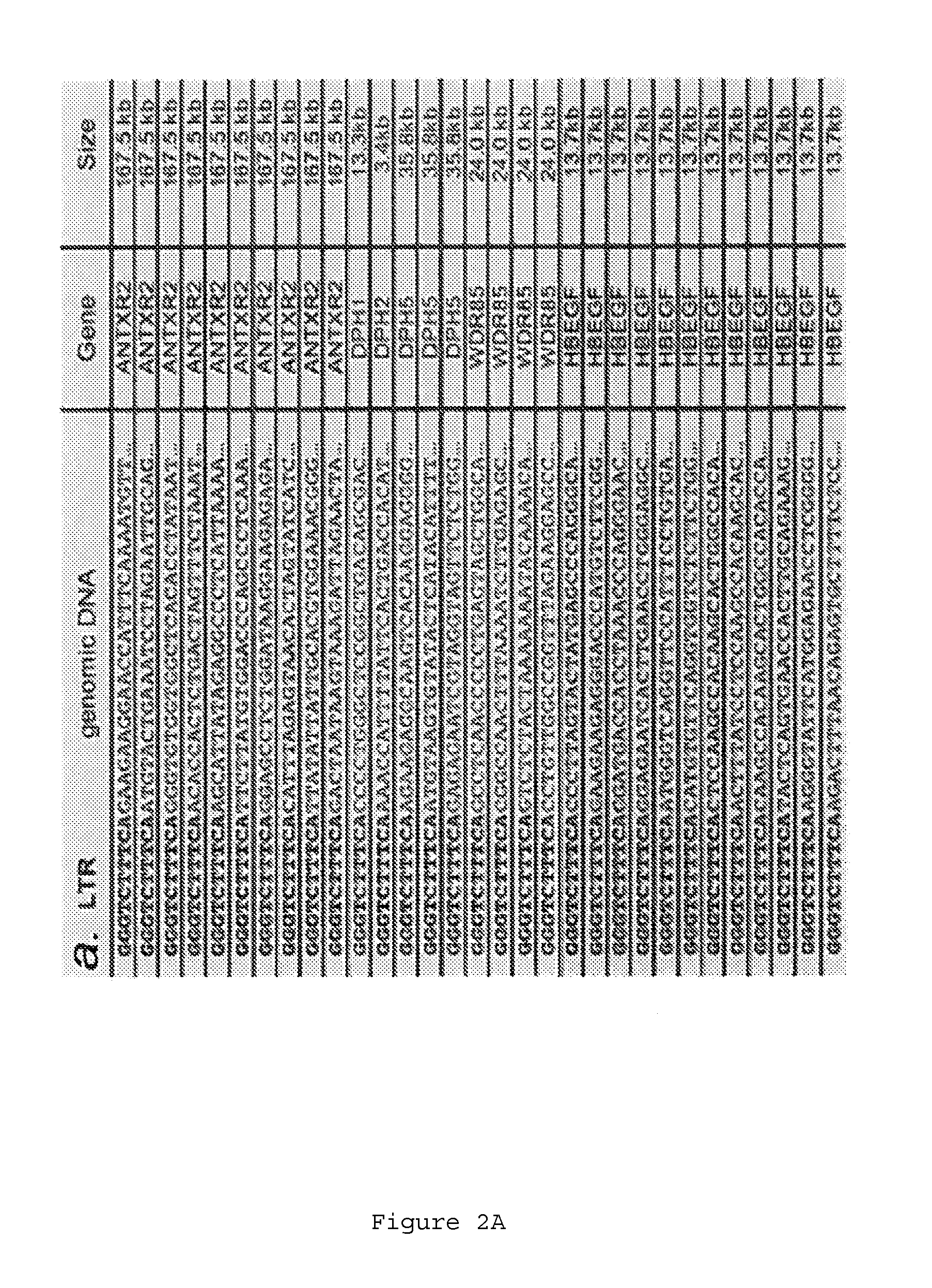
[0151]We next determined whether the observed haploid nature of the great majority of the genome sequence in the KBM7 subclone allowed the generation of knockout cells by mutagenesis. Since we knew that the cells could be readily infected by retroviruses, we explored the use in these cells of several viral gene-trap vectors designed to trap expressed genes. The large majority of promotorless gene trap vectors described in the literature are based on neomycin (G418, geneticin) selection. Surprisingly, initial experiments showed that KBM7 cells were inherently resistant to very high concentrations of neomycin, precluding use of preexisting vectors. A potentia exception was the UPA-Trap vector (described in Shigeoka et al. 2005 Nucleic Acids Res. 2005 33(2):e20.) that in addition to neomycin contains GFP. To test this vector, virus was produced by transfection of this vector with retroviral packaging vectors in 293...
example 3
Construction of Gene Trap Vectors Containing Vectors Containing Puromycin and GFP Selectable Markers
[0152]Novel retroviral gene trap vectors that contain an inactivated LTR, a strong splice-acceptor site derived from the long fiber gene of Adenovirus serotype 40 (Carette et al. 2005 The Journal of Gene Medicine 7(8) 1053-1062), and either GFP or the puromycin resistance gene (PURO) followed by a SV40 polyadenylation signal were constructed as follows. The coding sequence of the PURO or GFP was obtained by PCR amplification with primers containing overhanging ClaI and NheI restriction sites as well as partial splice acceptor sites: (GFP:5′-GATCGCTAGCCGCATTTCTTTTTTCCAGATGGTGAGCAAGGGCGAGG-3′ (SEQ ID NO: 126) and 5′-GATCGGATCCTTACTTGTACAGCTCGTCCATGC-3′ (SEQ ID NO: 127) PURO: 5′-GATCGCTAGCCGCATTTCTTTTTTCCAGATGACCGAGTACAAGCCCAC-3′ (SEQ ID NO: 128) and 5′-GATCGGATCCTCAGGCACCGGGCTTGCGGGTC-3′ (SEQ ID NO:129)). These PCR products were inserted in pEGFPC1(Clontech) replacing EGFP. Subsequently...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com