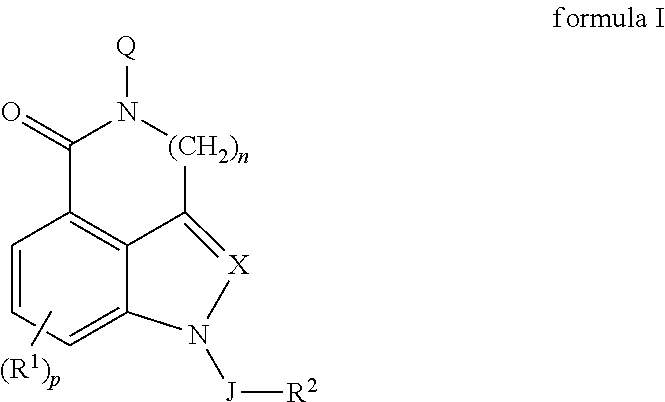
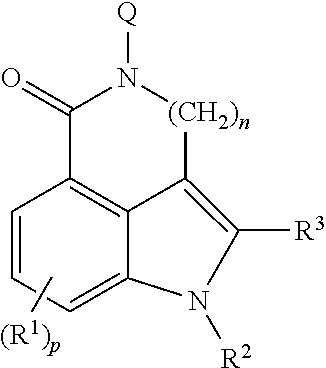
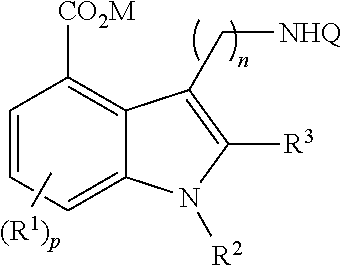
5-ht3 receptor modulators, methods of making, and use thereof
a technology of receptor modulators and serotonin, applied in the field of serotonin type 3 (5ht3) receptor modulators, can solve the problems of patients refusing further chemotherapy, unfavorable consequences, burden on the medical system, etc., and achieves the effect of inhibiting serotonin-induced bradycardia and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Methyl 1-methyl-1H-indole-4-carboxylate (A1a)
[0154]
Conditions: A) NaH, CH3I, DMF
[0155]Step A: To a stirring suspension of sodium hydride (60% dispersion in mineral oil, 9.90 g, 248 mmol) in DMF (150 mL) was slowly added a solution of methyl indole-4-carboxylate (10.0 g, 62.1 mmol) in DMF (100 mL) at room temperature under an atmosphere of nitrogen. The mixture stirred for 30 minutes, then iodomethane (15.4 mL, 248 mmol) was added and the mixture continued to stir at room temperature for an additional 16 hours. The mixture was quenched with a saturated solution of ammonium chloride (500 mL) and the aqueous mixture was extracted with ethyl acetate (3×300 mL). The combined organic layers were washed with water (4×300 mL) and brine (200 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. Purification of the resulting residue by column chromatography (0% to 30% ethyl acetate in hexanes) afforded methyl 1-methyl-1H-indole-4-carboxylate (A1a, 10.56 g, 90%...
example 2
Preparation of Methyl 1-benzyl-1H-indole-4-carboxylate (A1b)
[0156]
Conditions: A) BnBr, NaH or Cs2CO3, DMF
[0157]Step A: To a solution of methyl indole-4-carboxylate (10.0 g, 57.14 mmol) was added sodium hydride (60% dispersion in mineral oil (5.71 g, 142.8 mmol) in DMF (200 mL) in portions. The mixture was stirred under an atmosphere of nitrogen for 20 minutes. To this was added benzyl bromide (8.48 mL, 74.4 mmol) and the mixture continued to stir for 16 hours. The mixture was poured into an ice / water mixture and extracted with diethyl ether (3×500 mL). The combined organic layers were washed with brine (3×200 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. Purification by column chromatography (silica gel, 5 to 15% ethyl acetate in hexanes) afforded methyl 1-benzyl-1H-indole-4-carboxylate (A1b, 13.89 g, 92%) as a pale yellow oil, which crystallized upon standing: 1H NMR (500 MHz, CDCl3) δ 7.90 (d, J=7.5 Hz, 1H), 7.46 (d, J=7.5 Hz, 1H), 7.30-7.25 (m, 4H), 7.20...
example 3
Preparation of Methyl 3-formyl-1H-indazole-4-carboxylate (B1)
[0158]
Conditions: A) NaNO2, 6 N HCl, H2O
[0159]Compound B1, where R1═H was prepared using a modified procedure reported in patent WO02044183A2.
[0160]Step A: Aqueous HCl (56.0 mL of 6N solution in water, 0.33 mol) was added dropwise over 1 hour to a stirring suspension of methyl 1H-indole-4-carboxylate (5.0 g, 28.5 mmol) in a solution of sodium nitrite (24.0 g, 0.34 mol) in water (500 mL) at ambient temperature. The mixture was stirred overnight at ambient temperature, and then extracted with ethyl acetate (5×300 mL). The combined organic layers were washed with water (2×300 mL), brine (200 mL), and dried (Na2SO4). The organics were concentrated under reduced pressure until precipitation was observed. After cooling in a dry ice bath the precipitate was collected by filtration, washed with cold ethyl acetate (50 mL), and hexanes (100 mL) and dried (Na2SO4) to afford methyl 3-formyl-1H-indazole-4-carboxylate (B1, 2.1 g, 35%) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com