Prolonging Survival of Platelets Using CMP-Sialic Acid, UDP-Galactose or Both
a technology of cmpsialic acid and udpgalactose, which is applied in the field of prolonging the survival of platelets using cmpsialic acid, udpgalactose or both, can solve the problems of concomitant loss of hemostatic function, storage lesion defects, etc., and achieve the effect of reducing the incidence of platelet clearan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Introduction
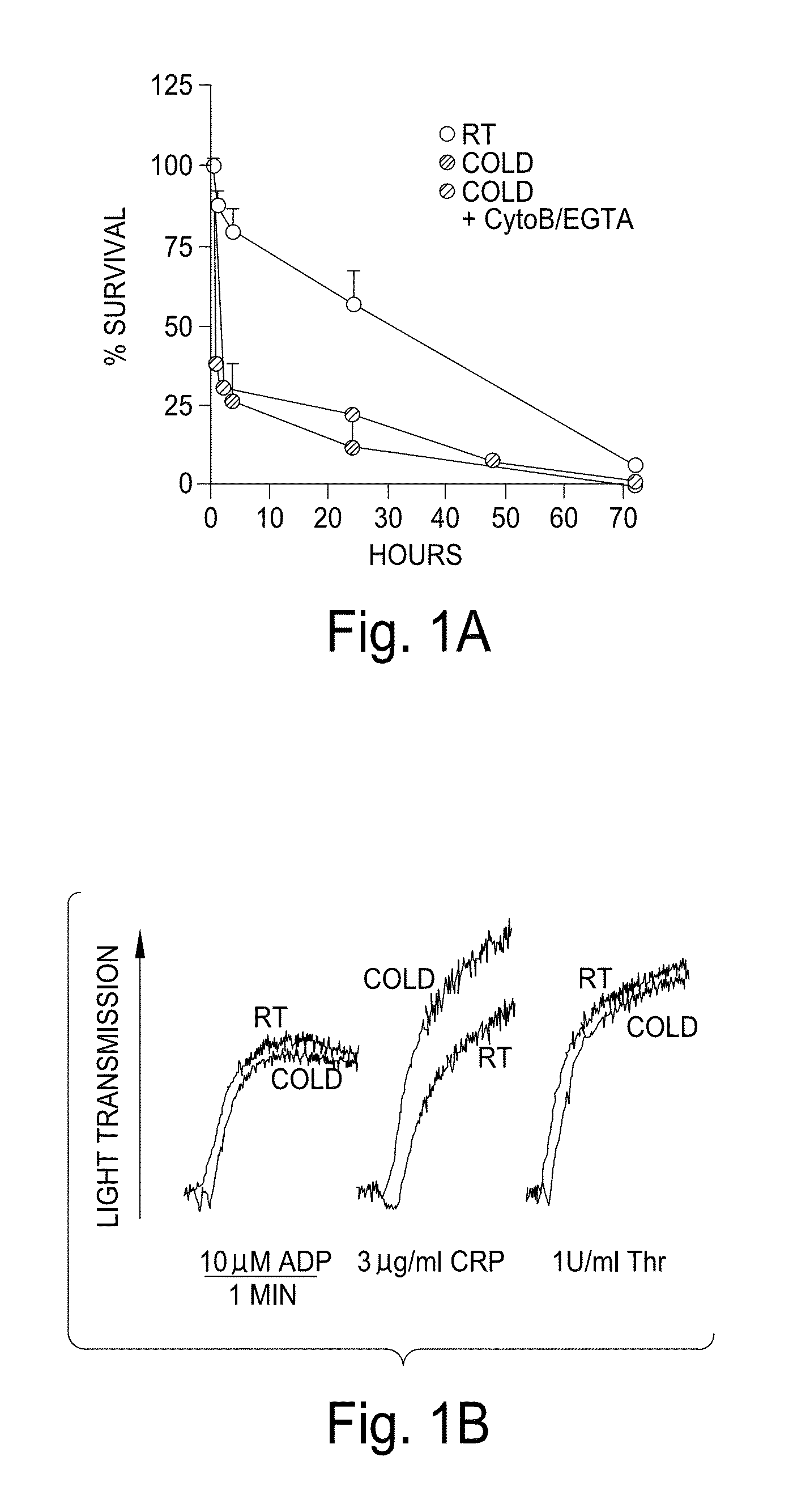
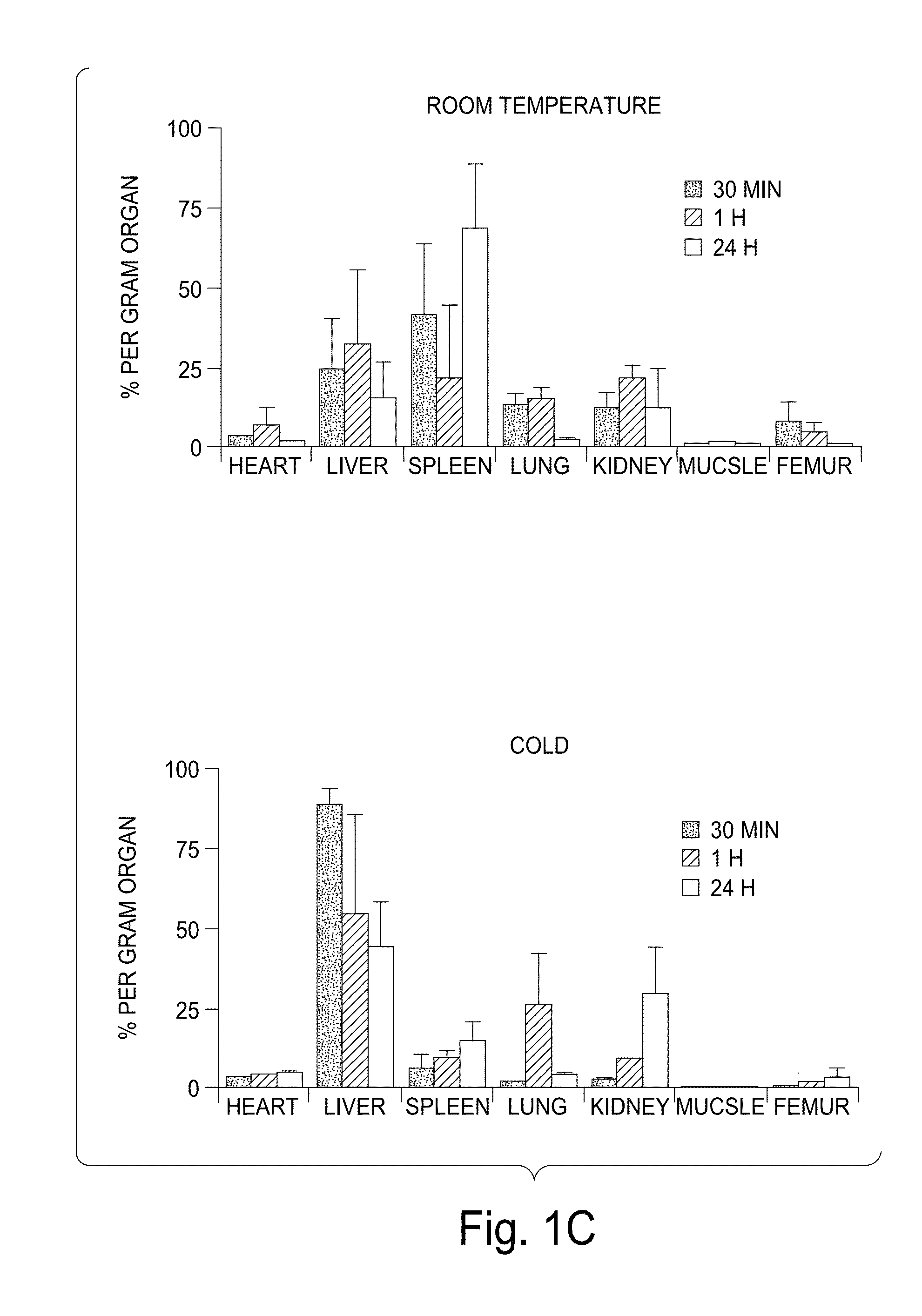
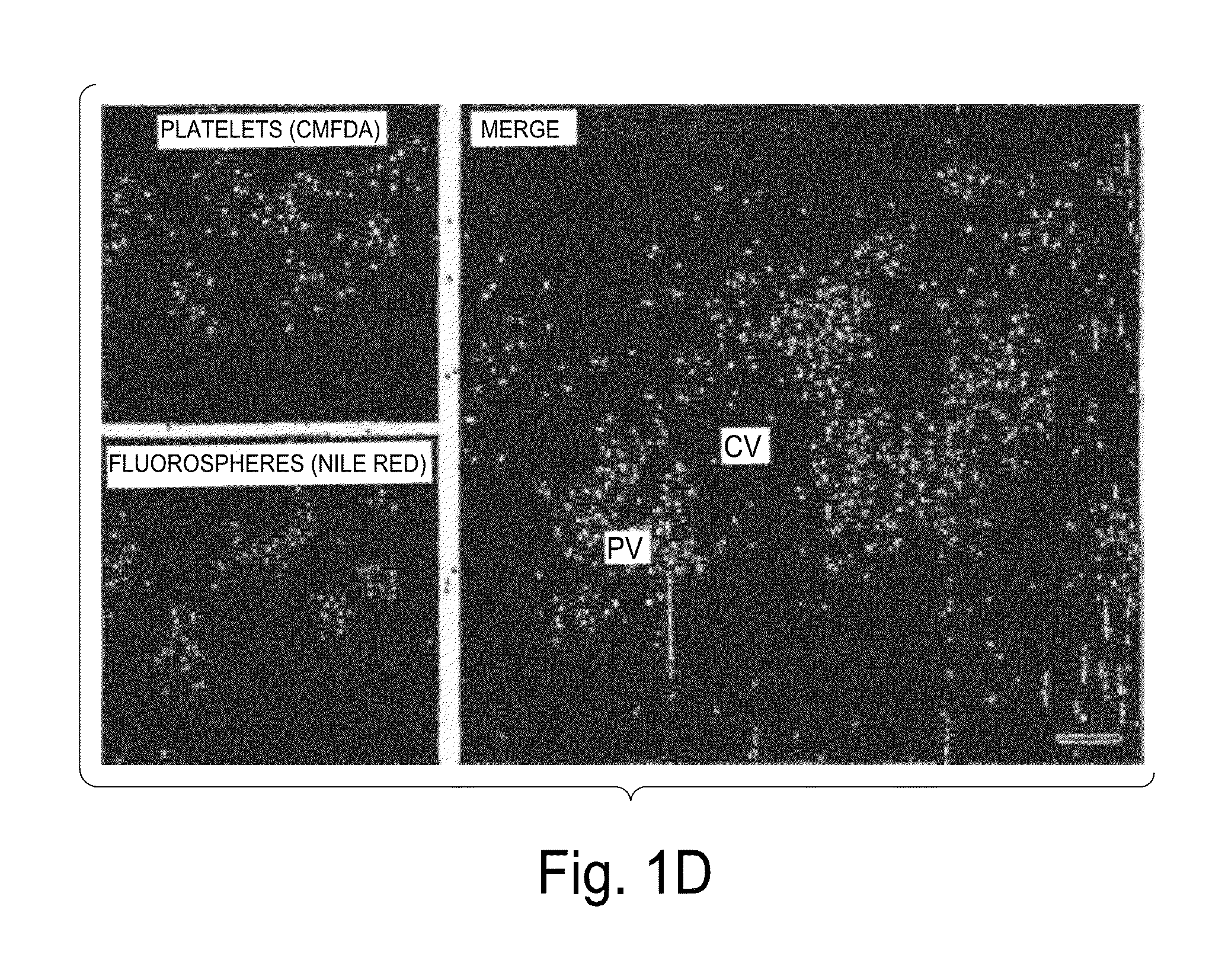
[0166]Modest cooling primes platelets for activation, but refrigeration causes shape changes and rapid clearance, compromising storage of platelets for therapeutic transfusions. We found that shape change inhibition does not normalize cold-induced clearance. We also found that cooling platelets rearranges the surface configuration of the von Willebrand factor (vWf) receptor complex α subunit (GP1bα) such that it becomes targeted for recognition by complement receptor 3 receptors (CR3) predominantly expressed on liver macrophages, leading to platelet phagocytosis and clearance. GP1bα removal prolongs survival of unchilled platelets. Chilled platelets bind vWf and function normally in vitro and ex vivo after transfusion into CR3-deficient mice. Cooled platelets, however, are not “activated” like platelets exposed to thrombin or ADP, and their vWf-receptor complex reacts normally with activated vWf.
[0167]As the temperature falls below 37° C. platelets become more susceptibl...
example 2
Implication of the αMβ2 (CR3) Lectin Domain in Chilled Platelet Phagocytosis
[0256]αMβ2 (CR3) has a cation-independent sugar-binding lectin site, located “C-T” to its I-domain (Thornton et al, J. Immunol. 156, 1235-1246, 1996), which binds to mannans, glucans and N-Acetyl-D-glucosamine (GlcNAc). Since CD16b / αMβ2 membrane complexes are disrupted by β-glucan, N-Acetyl-D-galactosamine (GalNAc), and methyl-α-mannoside, but not by other sugars, it is believed that this interaction occurs at the lectin site of the αMβ2 integrin (CR3) (Petty et al, J. Leukoc. Biol. 54, 492-494, 1993; Sehgal et al, J. Immunol. 150, 4571-4580, 1993).
[0257]The lectin site of αMβ2 integrin has a broad sugar specificity (Ross, R. Critical Reviews in Immunology 20, 197-222, 2000). Although sugar binding to lectins is usually of low affinity, clustering can cause a more robust interaction by increasing avidity. The clustering of GP1bα following cooling, as shown by electron microscopy, suggests such a mechanism. T...
example 3
Enzymatic Modification of Platelet β-Glycans Inhibit Phagocytosis of Cooled Platelets by Macrophages In Vitro and Accommodate Normal Circulation In Vivo
[0273]Our preliminary experiments have demonstrated the enzymatic covering of GlcNAc residues on GP1bα using galactose-transfer (glycan modification) onto chilled human platelet surfaces greatly reduced their in vitro phagocytosis. One interpretation of these findings is that GP1bα structure is altered on the surface of chilled human and murine platelets. This causes the exposure or clustering of GlcNAc, which is recognized by the lectin binding domain of αMβ2 leading to platelet removal. β-GlcNAc exposure can be measured by WGA binding and possibly by binding of recombinant αMβ2 lectin domain peptides. Resting human platelets bind WGA, which increases greatly after chilling. We propose that galactose transfer (glycan modification) will prevent GP1bα's interaction with αMβ2-lectin but not with vWf. This modification (galactose transf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com