A method and system for cryopreservation to achieve uniform viability and biological activity
a cryopreservation and biological activity technology, applied in the field of cryopreservation process, can solve the problems of cell damage during cryopreservation, significant loss of cell viability, and 80% or more loss of cell activity and viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Controlling the Nucleation Temperature
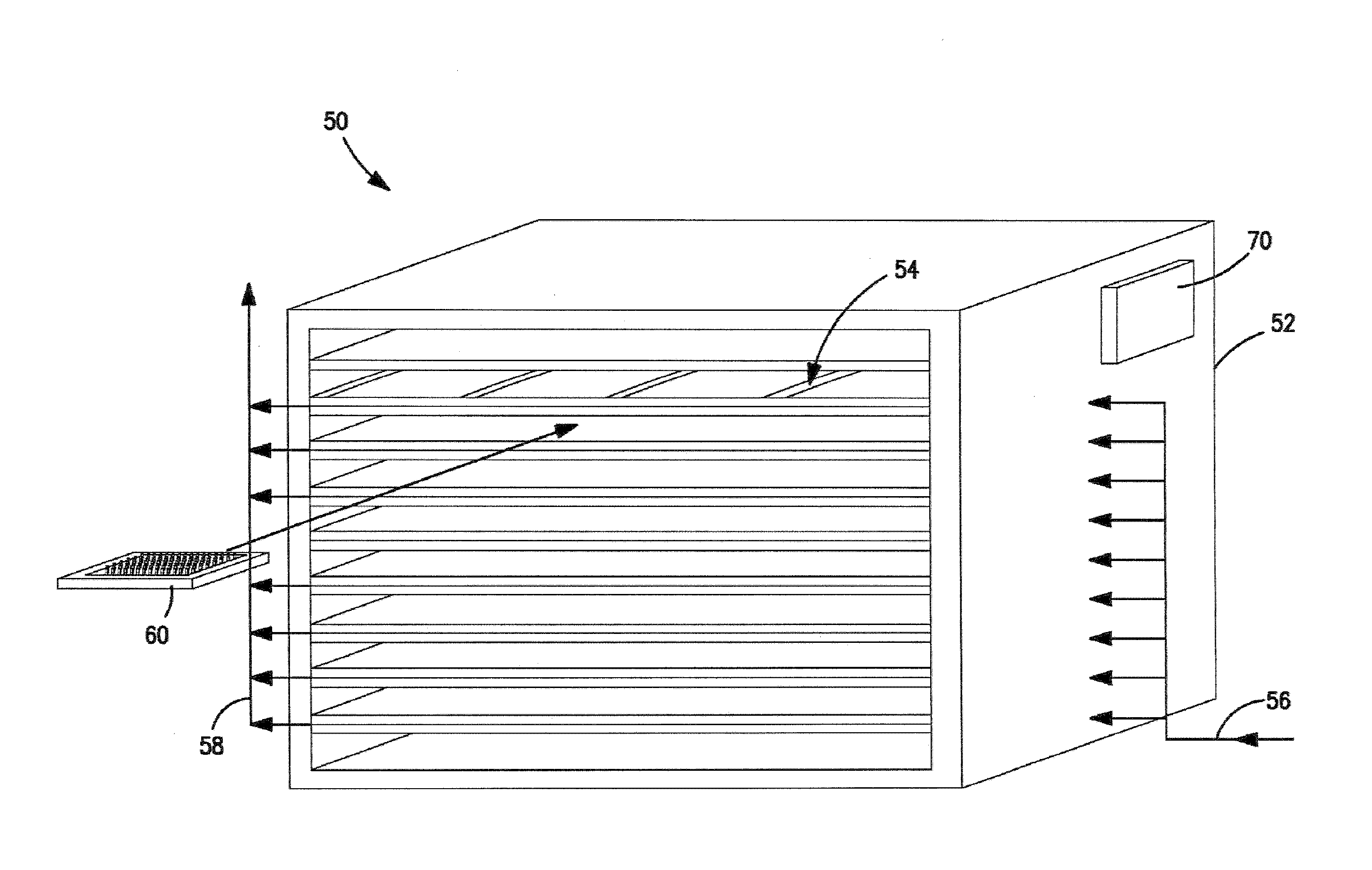
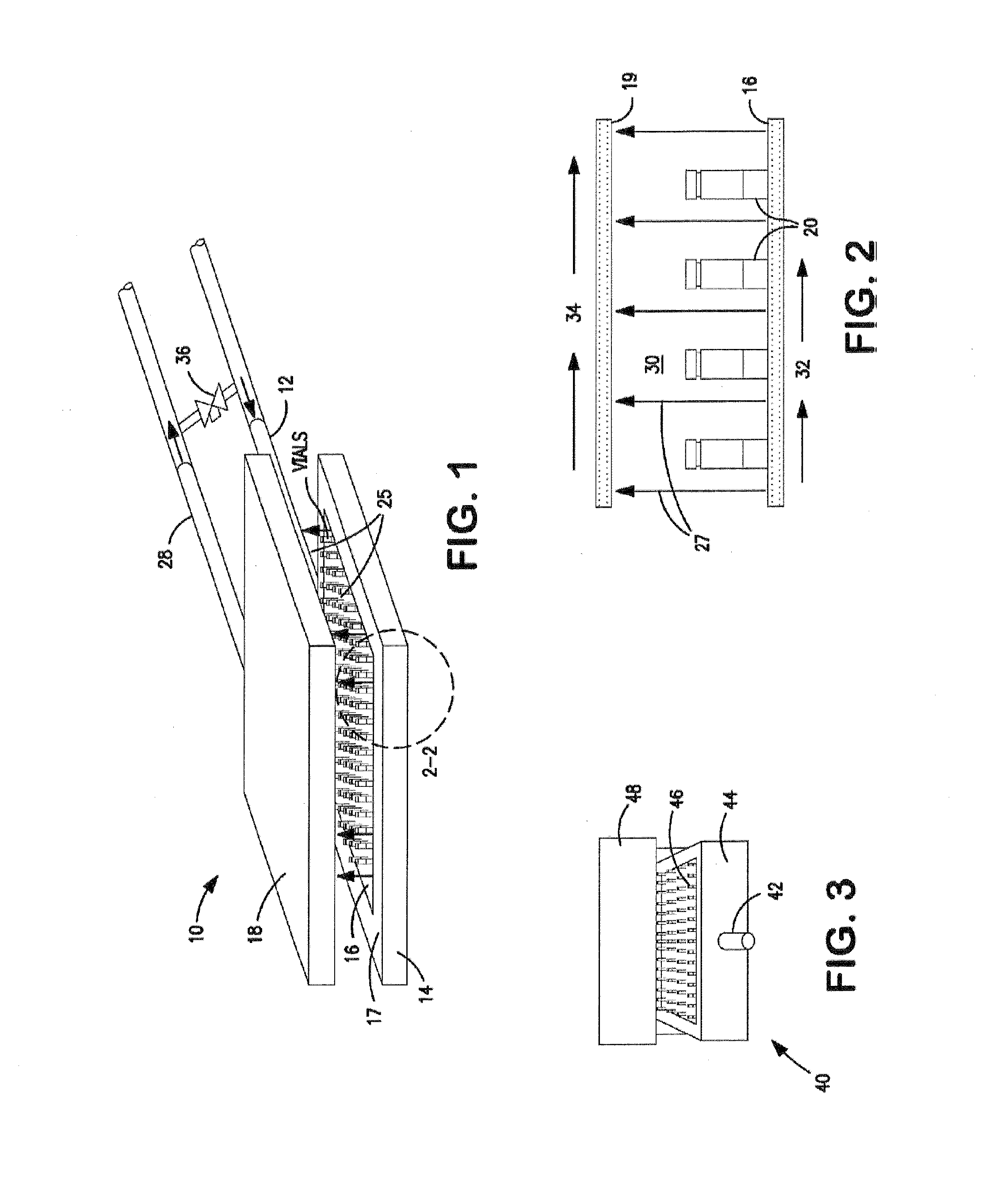
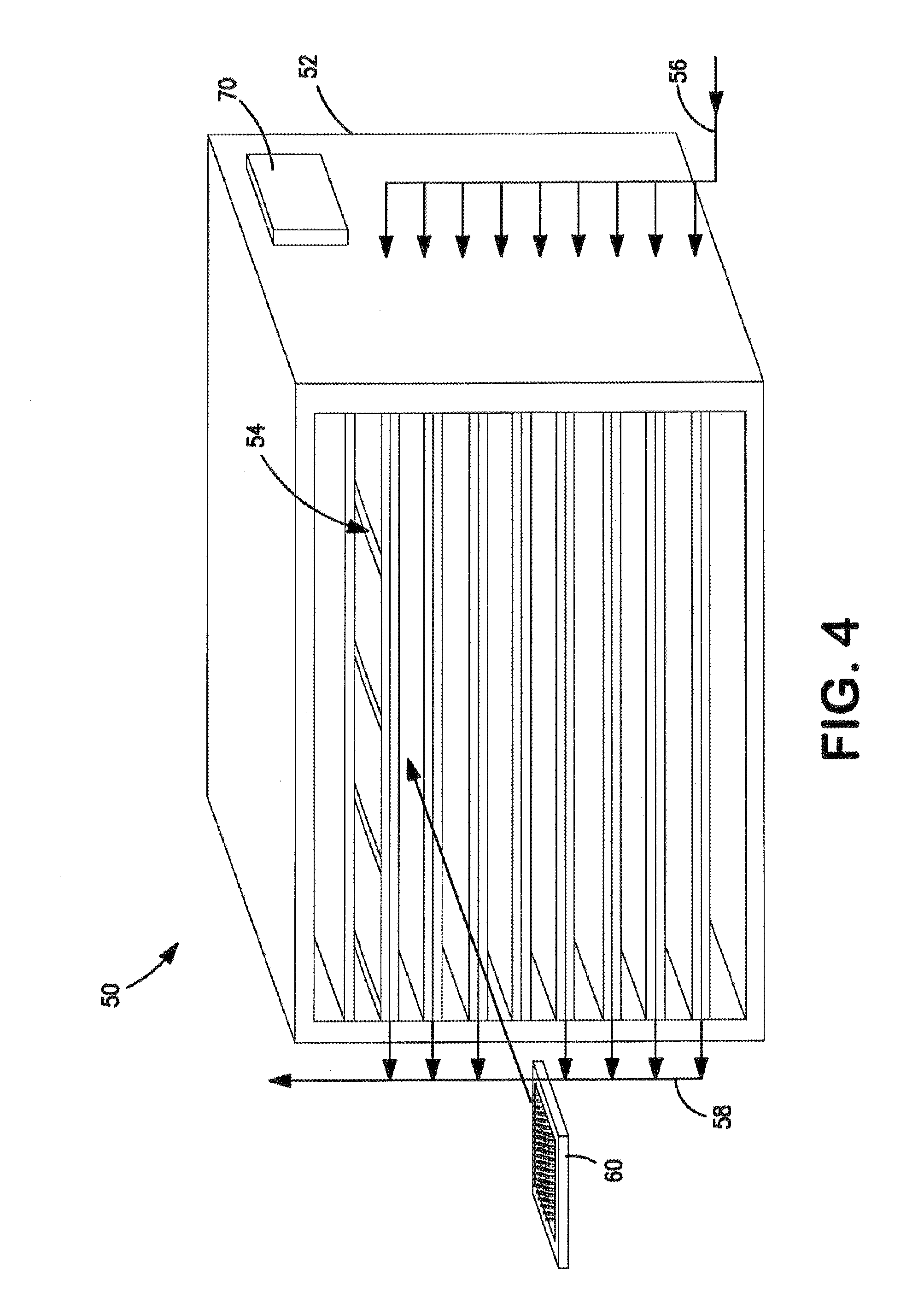
[0172]Four separate vials were filled with 2.5 mL of 5 wt % mannitol solution. The predicted thermodynamic freezing point of the 5 wt % mannitol solution is approximately −0.5° C. The four vials were placed on a freeze-dryer shelf in close proximity to one another. The temperatures of the four vials were monitored using surface mounted thermocouples. The freeze-dryer was pressurized with argon to 14 psig.
[0173]The freeze-dryer shelf was cooled to obtain vial temperatures of between approximately −1.3° C. and about −2.3° C. (+1° C. measurement accuracy of the thermocouples). The freeze-dryer was then depressurized from about 14 psig to about atmospheric pressure in less than five seconds to induce nucleation of the solution within the vials. All four vials nucleated and began freezing immediately after depressurization. Results are summarized in Table 1 below.
[0174]As seen in Table 1, the controlled nucleation temperatures in this example (i.e., ...
example 2
Controlling the Nucleation Temperature
[0175]In this example, ninety-five vials were filled with 2.5 mL of 5 wt % mannitol solution. The thermodynamic freezing point of the 5 wt % mannitol solution is approximately −0.5° C. The ninety-five vials were placed on a freeze-dryer shelf in close proximity to one another. The temperatures of six vials positioned at different locations in the freeze-dryer shelf were continuously monitored using surface mounted thermocouples. The freeze-dryer was pressurized in an argon atmosphere to about 14 psig. The freeze-dryer shelf was then cooled to obtain vial temperatures of near −5° C. The freeze-dryer was then depressurized from about 14 psig to about atmospheric pressure in less than five seconds to induce nucleation of the solution within the vials. All ninety-five vials were visually observed to nucleate and begin freezing immediately after depressurization. Thermocouple data for the six monitored vials confirmed the visual observation. The resu...
example 3
Controlling the Depressurization Magnitude
[0177]In this example, multiple vials were filled with 2.5 mL of 5 wt % mannitol solution. Again, the predicted thermodynamic freezing point of the 5 wt % mannitol solution is approximately −0.5° C. For each test run, the vials were placed on a freeze-dryer shelf in close proximity to one another. As with the earlier described examples, the temperatures of vials were monitored using surface mounted thermocouples. The argon atmosphere in the freeze-dryer was pressurized to differing pressures and the freeze-dryer shelf was cooled to obtain vial temperatures of about −5° C. In each test run, the freeze-dryer was then rapidly depressurized (i.e., in less than five seconds) from the selected pressure to atmospheric pressure in an effort to induce nucleation of the solution within the vials. Results are summarized in Table 3.
[0178]As seen in Table 3, the controlled nucleation occurred where the pressure drop was about 7 psi or greater and the nuc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com