Protein binding domains stabilizing functional conformational states of gpcrs and uses thereof
a technology of functional conformational state and protein binding domain, which is applied in the field of gpcr structure biology and signaling, can solve the problems of biochemical instability and difficulty in obtaining high-resolution crystal structure, incompatible structural heterogeneity with crystal formation, and difficulty in obtaining active state of gpcr structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunization, Library Construction and Initial Screening
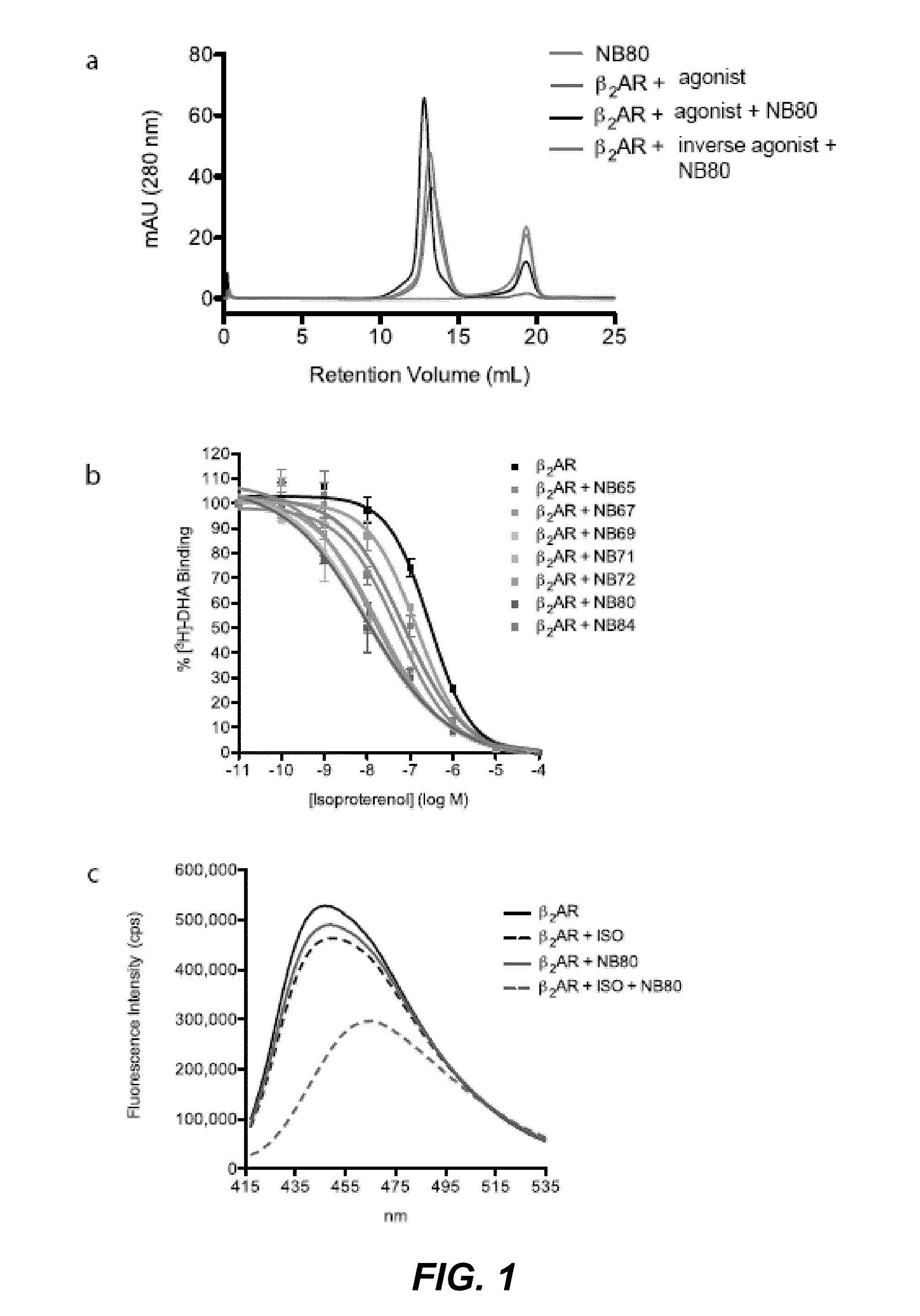
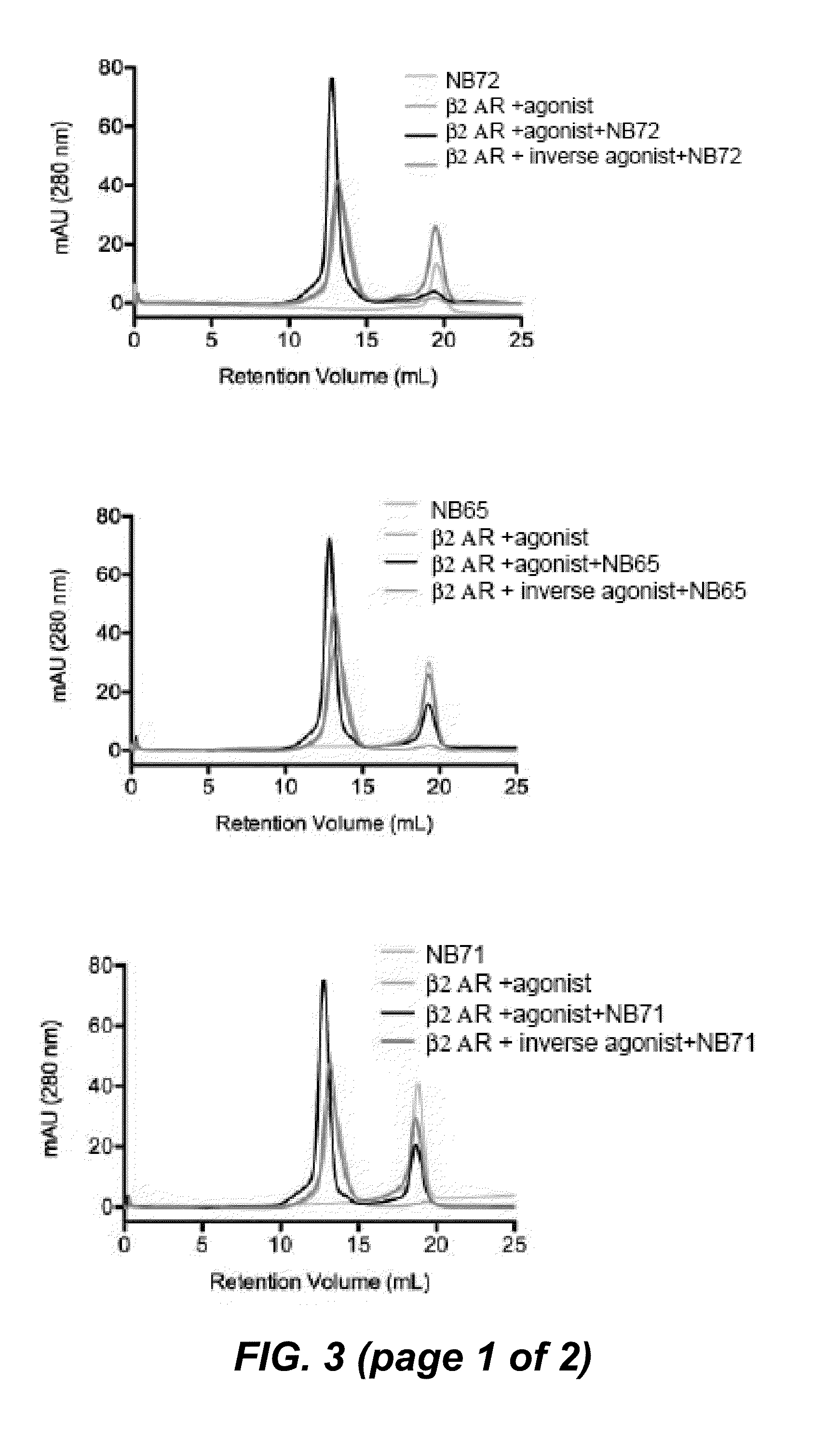
[0275]To obtain in vivo matured nanobodies against β2AR, a llama (Llama glama) was immunized with recombinant β2AR truncated at Gly365 (β2AR-365) to exclude an immune response to the carboxyl terminus. β2AR-365 was expressed in insect cells and antigen was reconstituted as previously described (Day et al. 2007). After six weekly administrations of the reconstituted truncated agonist-bound receptor, lymphocytes were isolated from the blood of the immunized llama and a phage library prepared and screened as described in Materials and Methods to the Examples (see further). Two screens identified conformational nanobodies that recognize the native β2AR, but not the denaturated receptor.
example 2
Selection of Conformational-Specific Nanobodies by ELISA
[0276]In a first screen we compared the binding of the nanobodies on the native and heat denatured β2AR antigen in an ELISA. For each nanobody, one well was coated with phospholipid vesicles containing agonist-bound β2AR-365 (0.1 μg protein / well). Next, this plate was incubated at 80° C. for two hours. Next, another well of the same plate was coated with phospholipid vesicles containing agonist-bound β2AR-365 (0.1 μg protein / well) without heating. All of the nanobodies were able to selectively bind the native receptor but not the heat inactivated receptor, indicating that 16 binders recognize conformational epitopes.
example 3
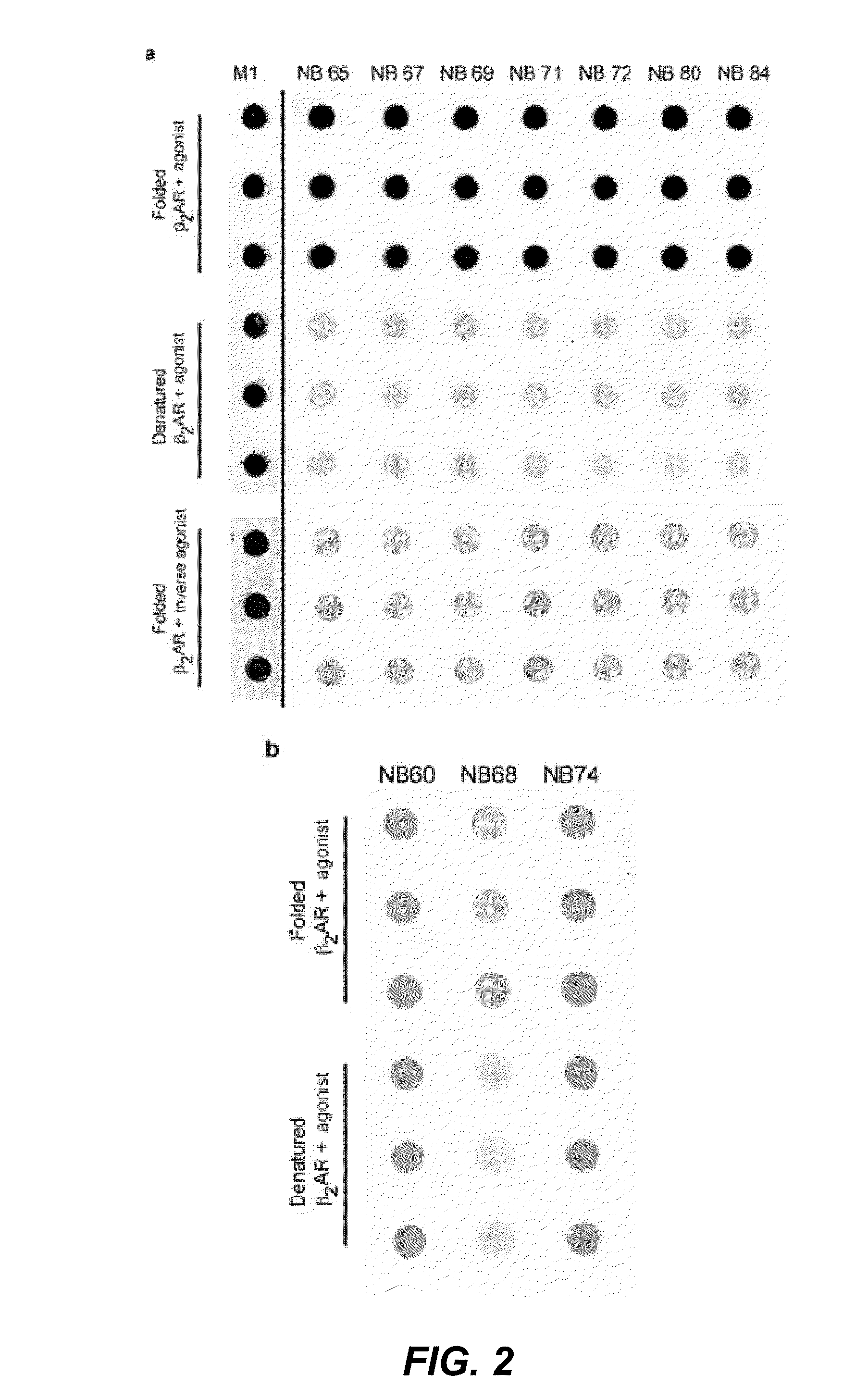
Selection of Conformational-Specific Nanobodies by Dot Blot
[0277]In a next screen we compared the specificity of the nanobodies for a native agonist-bound β2AR receptor, versus a native inverse agonist-bound receptor, versus an SDS denaturated receptor by dot blot analysis. The screen identified 16 different conformational nanobodies that recognize native agonist-bound β2AR-365, but not the inverse agonist, or the heat denatured receptor (FIG. 2 (dot blots)).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com