Acamprosate formulations, methods of using the same, and combinations comprising the same
a technology of acamprosate and a compound, applied in the field of acamprosate formulations, methods of using the same, and combinations comprising the same, can solve the problems of low bioavailability of bcs class iii compounds, low bindability of compounds, and reduced response of voltage-operated calcium channels to high levels of glutamate stimulation, etc., to achieve greater relief of anxiety and agitation, lower risk of tardive dyskinesia, and benefi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0127]Case 1: A 56-year old woman had long-standing tardive dyskinesia induced by treatment of schizoaffective disorder with a variety of neuroleptics and mood stabilizers. Her TD was characterized by side to side movements of the jaw, grimacing movements, rocking of the trunk, and continual involuntary kicking, leg-crossing, and twisting movements of her legs and feet. At the time she presented for treatment of her TD she was treated for her mental illness with lamotrigine and quetiapine, a second-generation neuroleptic. She was started on acamprosate 666 mg three times a day, with partial relief of symptoms. When acamprosate was increased to 999 mg three times a day she had complete relief of her TD. After two months free of symptoms of TD she switched from quetiapine to perphenazine, a first-generation neuroleptic; her TD symptoms did not return.
[0128]After additional weeks free of TD symptoms she discontinued the acamprosate. Her TD symptoms returned, as did feelings of anxiety ...
example 2
[0132]CASE 2: A 34-year old man had been treated with acamprosate for several years for TD due to exposure to several neuroleptics for schizoaffective disorder. He was currently treated with lamotrigine and quetiapine for his mental illness, and was taking acamprosate 1032 mg+999 mg+1032 mg on a three times daily basis. This dose of acamprosate completely relieved his involuntary movements of TD—the latter including involuntary movements of the cheeks and mouth, rocking movements of the trunk, and twisting movements of the both upper and lower extremities. 999 mg three times a day did not give full relief from his involuntary movements. To test the therapeutic threshold hypothesis the patient was asked to try 1032 mg of acamprosate once a day in the morning. On this dose he was free of movements in the morning and early afternoon but movements returned in the evening. When he added a second dose of 1032 mg in the late afternoon—8 to 10 hours after his first dose—he obtained complete...
example 3
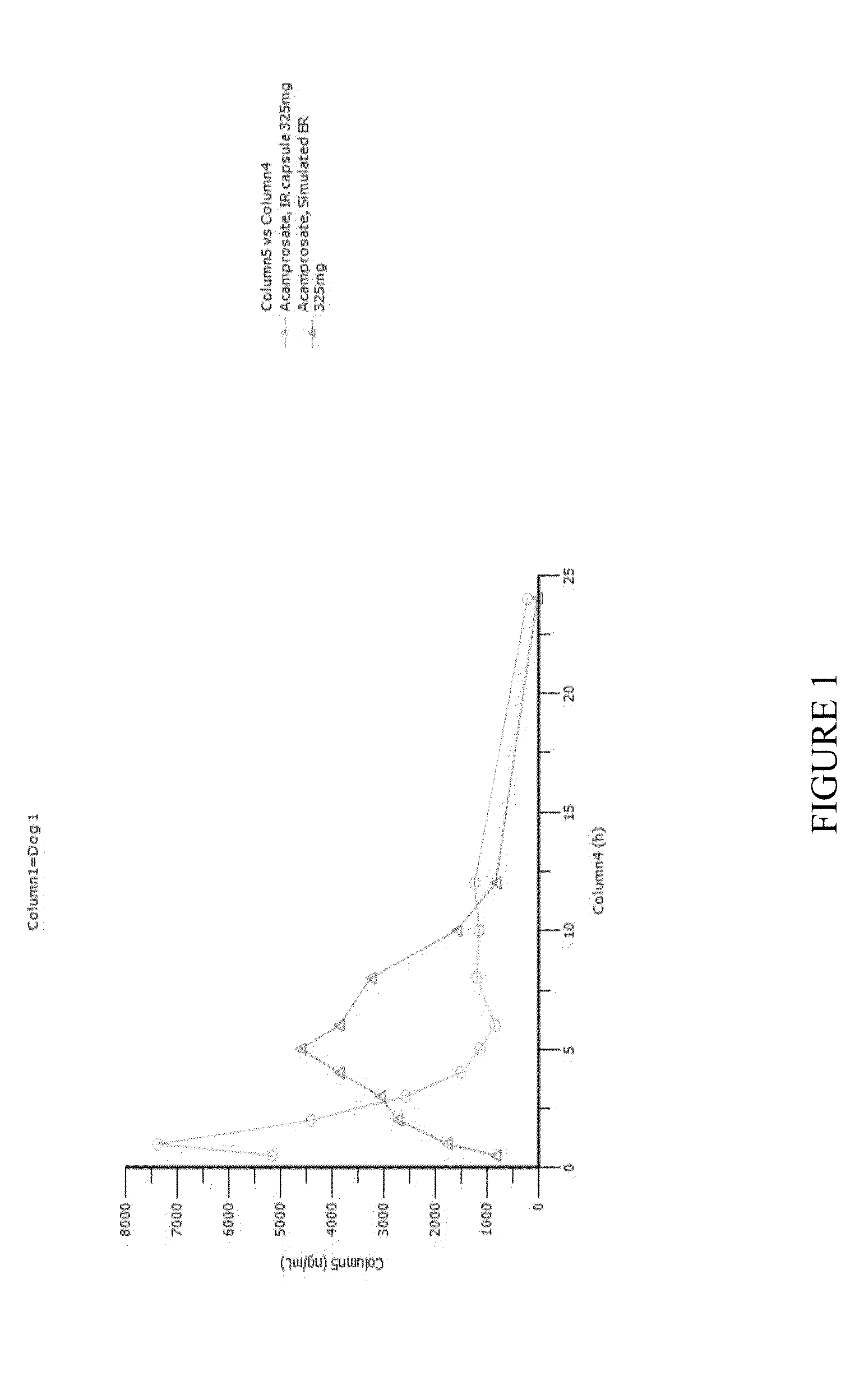
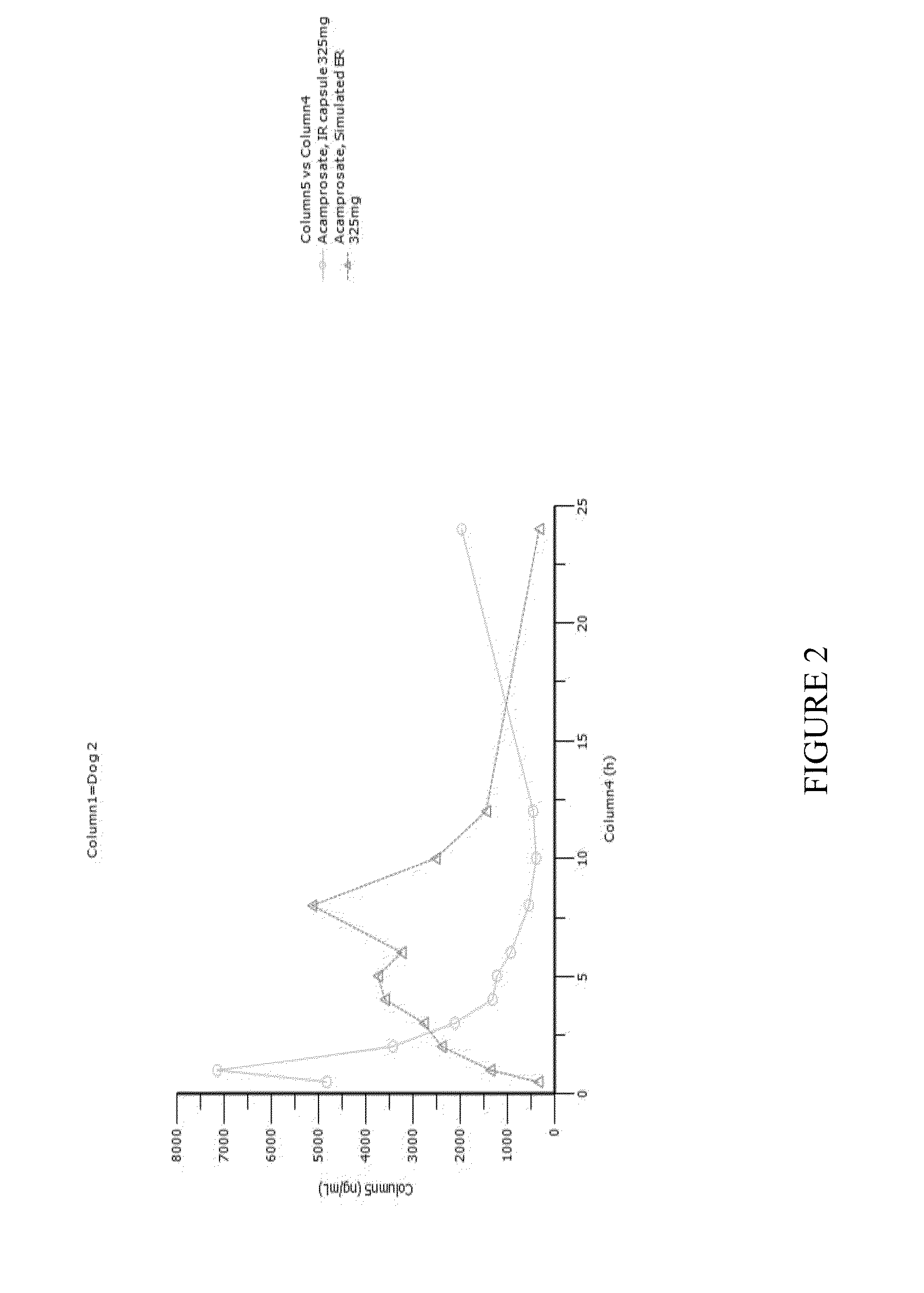
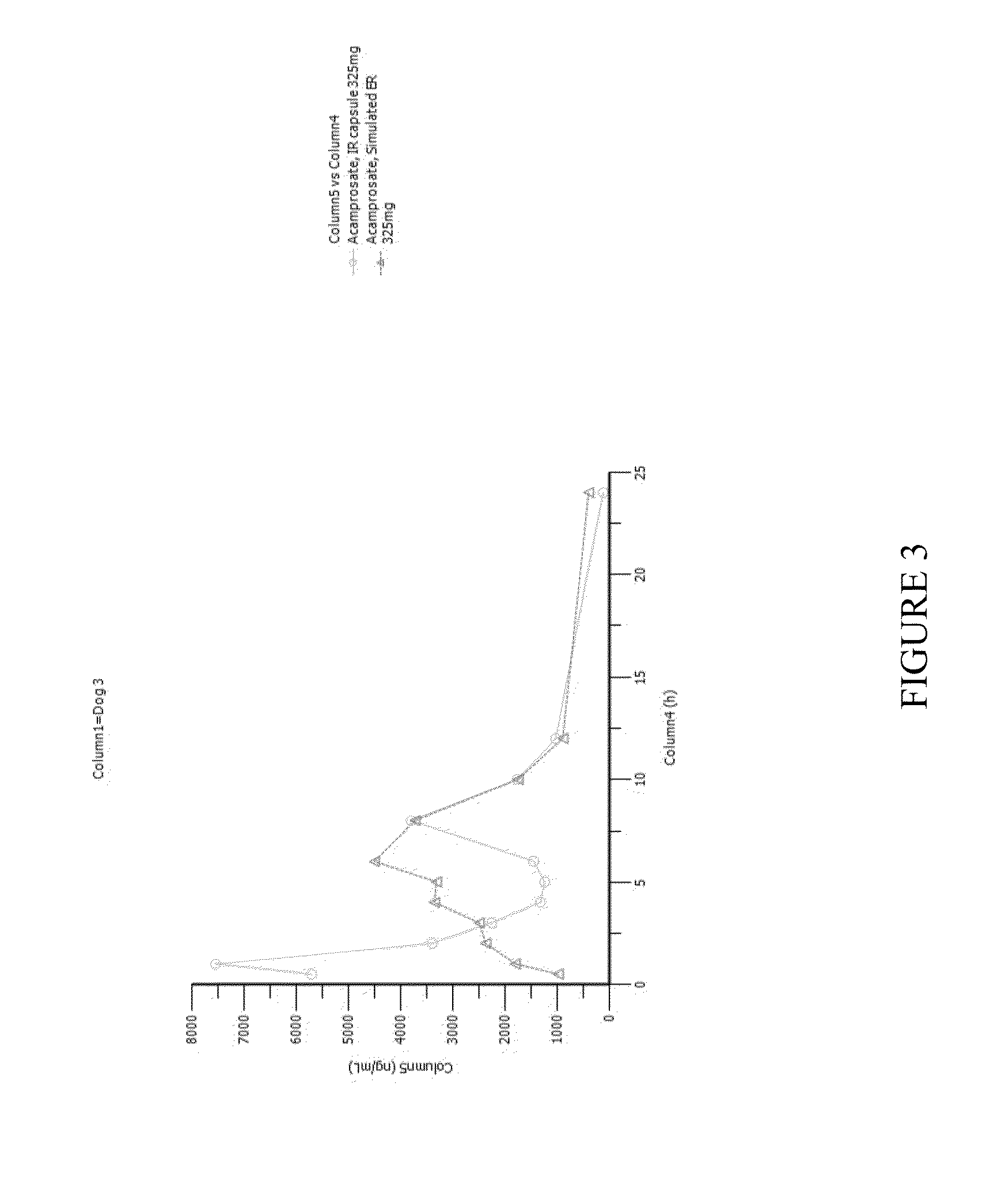
[0135]A pharmacokinetic study was conducted in four dogs. Dogs were given immediate-release (IR) acamprosate capsules orally. On one day they were given a single capsule containing 325 mg of acamprosate. On another day one week later the dogs were given 325 mg of acamprosate divided into smaller doses administered every 30 minutes, as shown in Table 1 below.
[0136]This mode of delivering acamprosate mimics the delivery of acamprosate into the stomach by a controlled-release GR system. FIGS. 1-4 are time-concentration curves for each of the dogs that compare IR acamprosate with simulated GR controlled-release acamprosate.
[0137]Table 2 shows pharmacokinetic parameters of the two delivery versions of acamprosate in each of the four dogs and displays ratios of interest between the two versions for several parameters of interest. In the table the residence time above two arbitrarily selected thresholds—2000 ng / mL and 3000 ng / mL was calculated by measuring the graphs; an asterisk next to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total weight | aaaaa | aaaaa |
| transit time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com