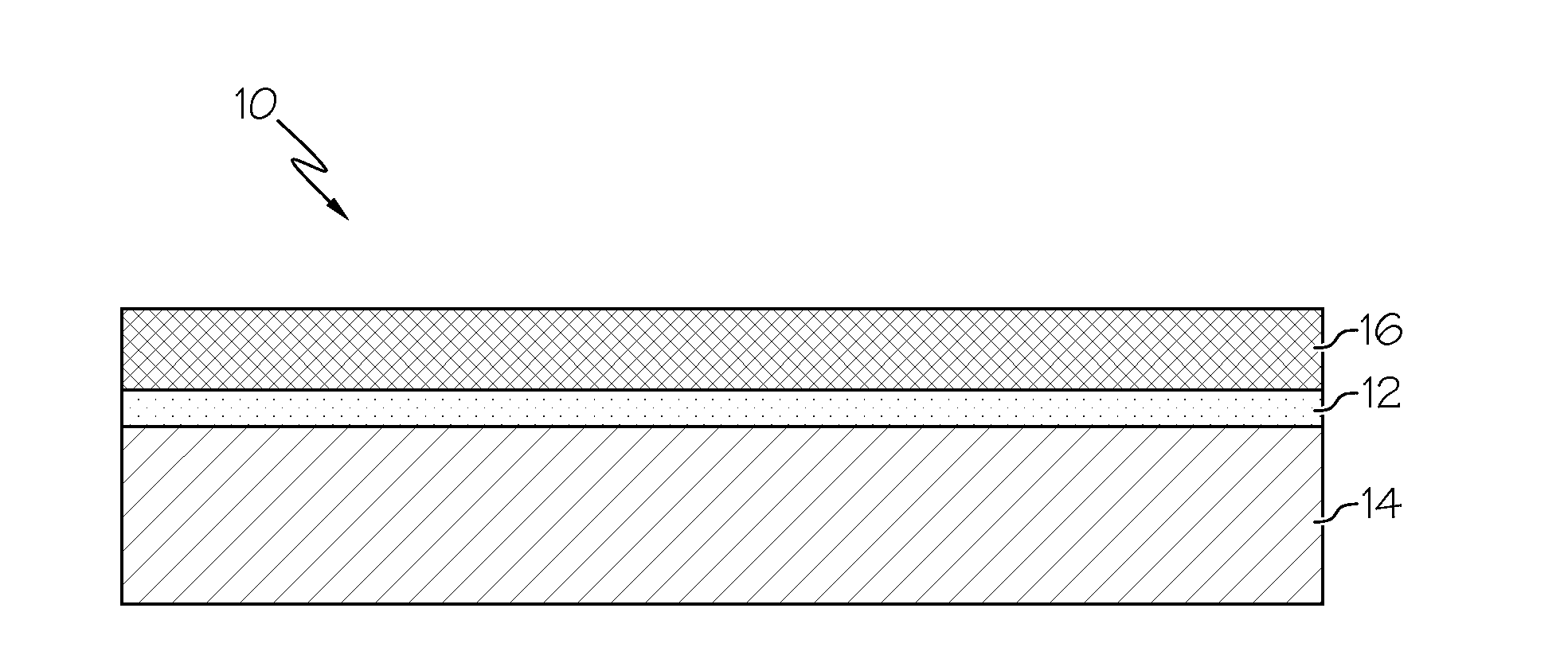
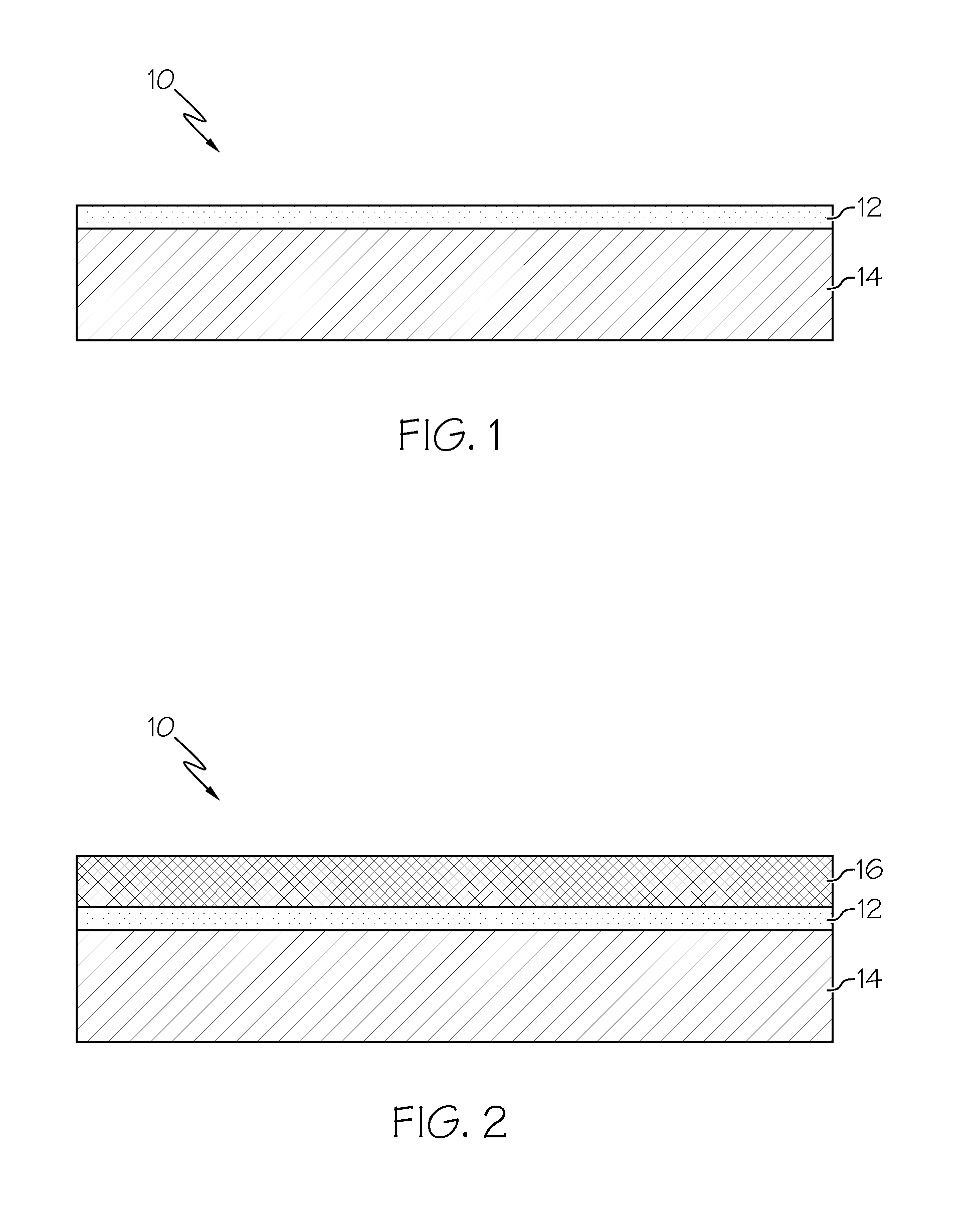
Exhaust treatment system including a nickel-based catalyst
a technology of exhaust treatment system and catalyst, which is applied in the direction of metal/metal-oxide/metal-hydroxide catalyst, machine/engine, arsenic compound, etc., can solve the problems that conventional cerium-based oxides do not generate the oxygen needed for hc and co oxidation, and achieve the reduction of carbon monoxide, hydrocarbon emissions, and nitrogen oxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0052]Samples with various Ni loadings on a cordierite carrier were prepared. A 2.15 g of nickel nitrate solution with 0.1013 g Ni / g-solution was prepared and impregnated into a 4.53 g cordierite substrate having a honeycomb structure of 1 inch×1 inch. The impregnated sample was then dried at 150° C. for 1 hour and calcined at 350° C. in air for one hour prior to use. The resulting substrate contained 4.6 wt % Ni. The OSC values for the sample were measured shortly after preparation by placing the sample in a quartz reactor with helium flowing through the reactor, followed by the addition of CO into the helium flow. The consumption of CO or the production of CO2 from the reaction of CO to oxygen contained in the OSC-bearing catalyst was monitored using a quadruple mass spectrometer. The OSC values are shown in Table 1 below.
TABLE 1OSC values for 4.6 wt % Ni on cordierite of 1″ L × 1″ DOSC (mole O) measured at ° C.Theoretical OSC400° C.600° C.700° C.3.73 × 10−3 mole O3.86 × 10−33.81 ...
example 2
[0053]A commercial catalyst containing 5 wt % nickel was prepared and subjected to a simulated aging process mimicking 4,000 (4K) or 120,000 (120K) vehicle miles. The samples were aged on a pulse-flame combustor (pulsator) using a Ford standard 4-mode aging cycle (the pulsator combusts fuel to generate a simulated vehicle exhaust). The samples were placed in the pulsator exhaust gas stream and subjected to cyclic air-fuel conditions at an exponentially weighted temperature of 930° C. in order to accelerate the aging process. The 4K samples were aged for 8 hours and the 120K samples were aged for 120 hours.
[0054]The OSC value of the commercial catalyst was determined using a gasoline flame-combustor (pulsator). These values were compared to catalysts prepared as described below containing 1) 12 wt % Ni on cordierite and 2) 12 wt % Ni and 1.7 wt % Pt.
[0055]Sample 1 was prepared by impregnating a substrate of 4.53 g cordierite (1″L×VD) with 2.15 g of Ni solution with 0.1013 g Ni / g-solu...
example 3
[0059]A catalyst was prepared by washcoating alumina onto a cordierite substrate and then impregnating the alumina / cordierite with nickel. The process of loading nickel was the same as that described in Example 1. The catalyst of nickel on alumina / cordierite the two samples of nickel on cordierite were aged under alternative flows of 1% CO and 0.5% O2 at 1000° C. (redox-aging) for 12 hours, simulating the rich-lean cycles of gasoline engine exhausts. The OSC values after the redox-aging are shown in Table 3.
TABLE 3OSC after the redox-aging at 1000° C. over 1′ L × 1″ D samples: alumina effectOSC (×10−3 mole O) measured at T =Ni loadingSupport600° C.700° C.13.8 wt %alumina / cordierite0.630.768.95 wt %cordierite3.045.1012.6 wt %cordierite4.757.96
[0060]As can be seen, the OSC values for nickel on alumina / cordierite after aging were dramatically diminished in comparison with those for the sample of nickel on cordierite. It is believed that the nickel-alumina interaction caused the severe ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com