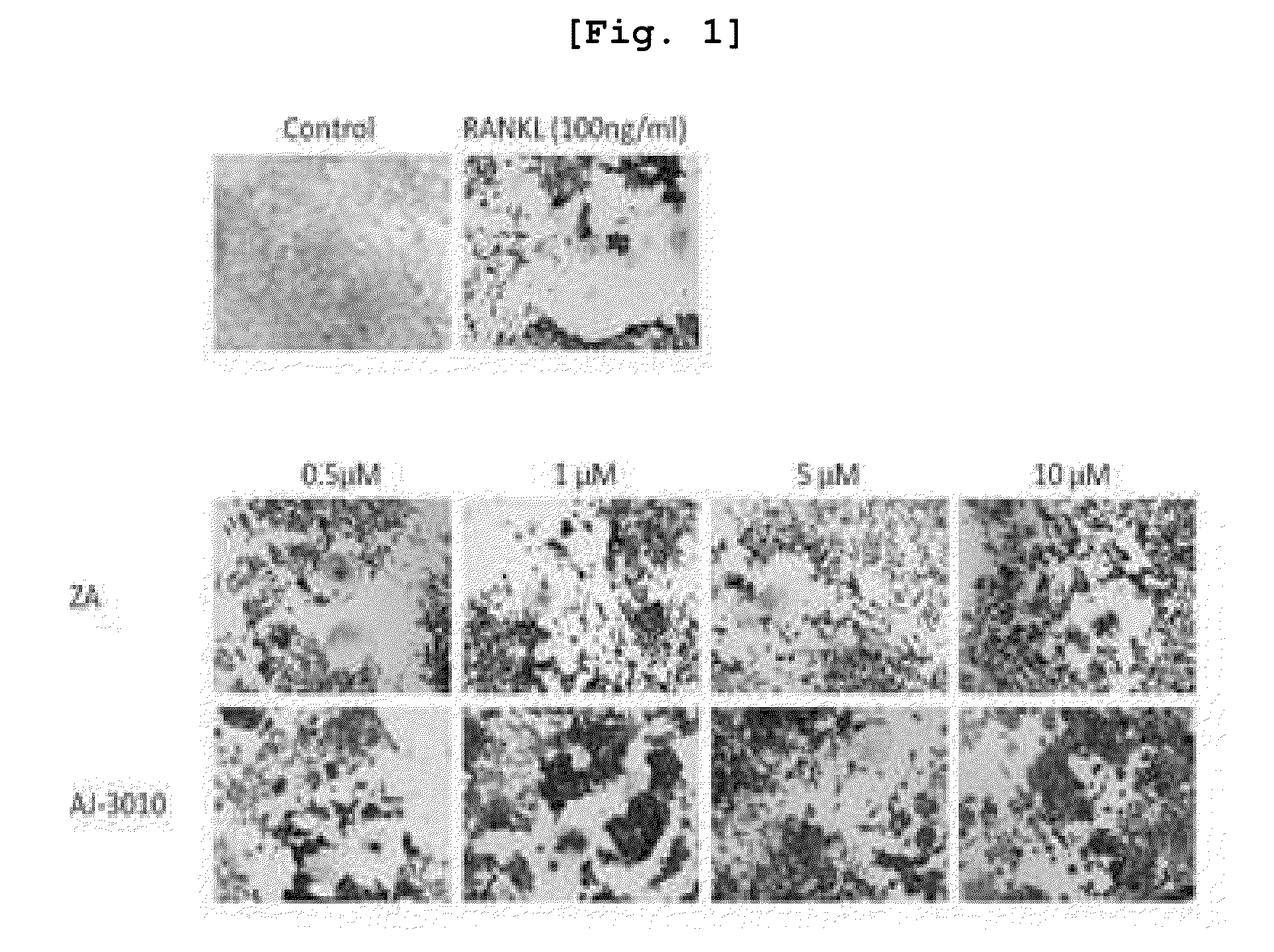
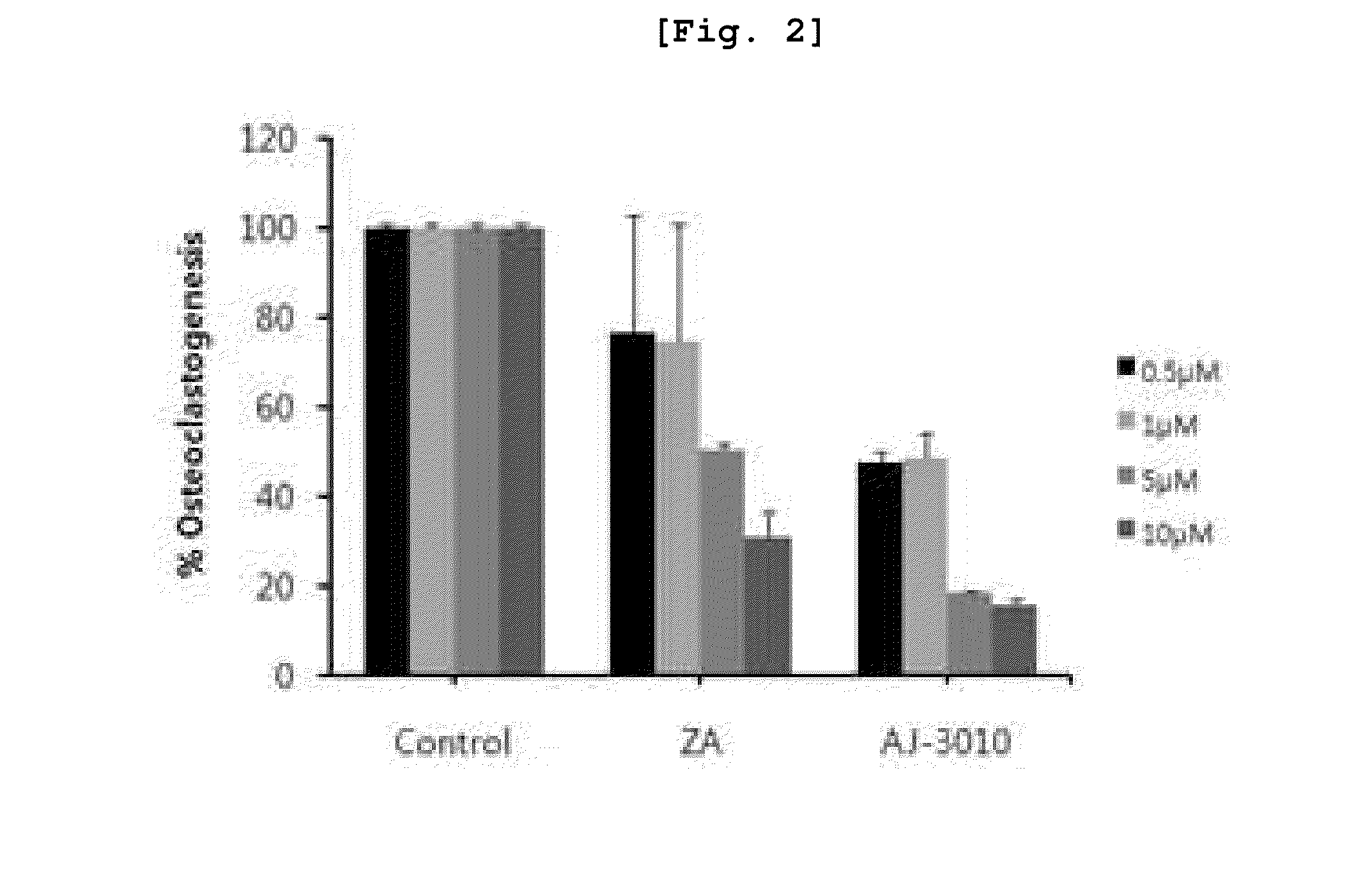
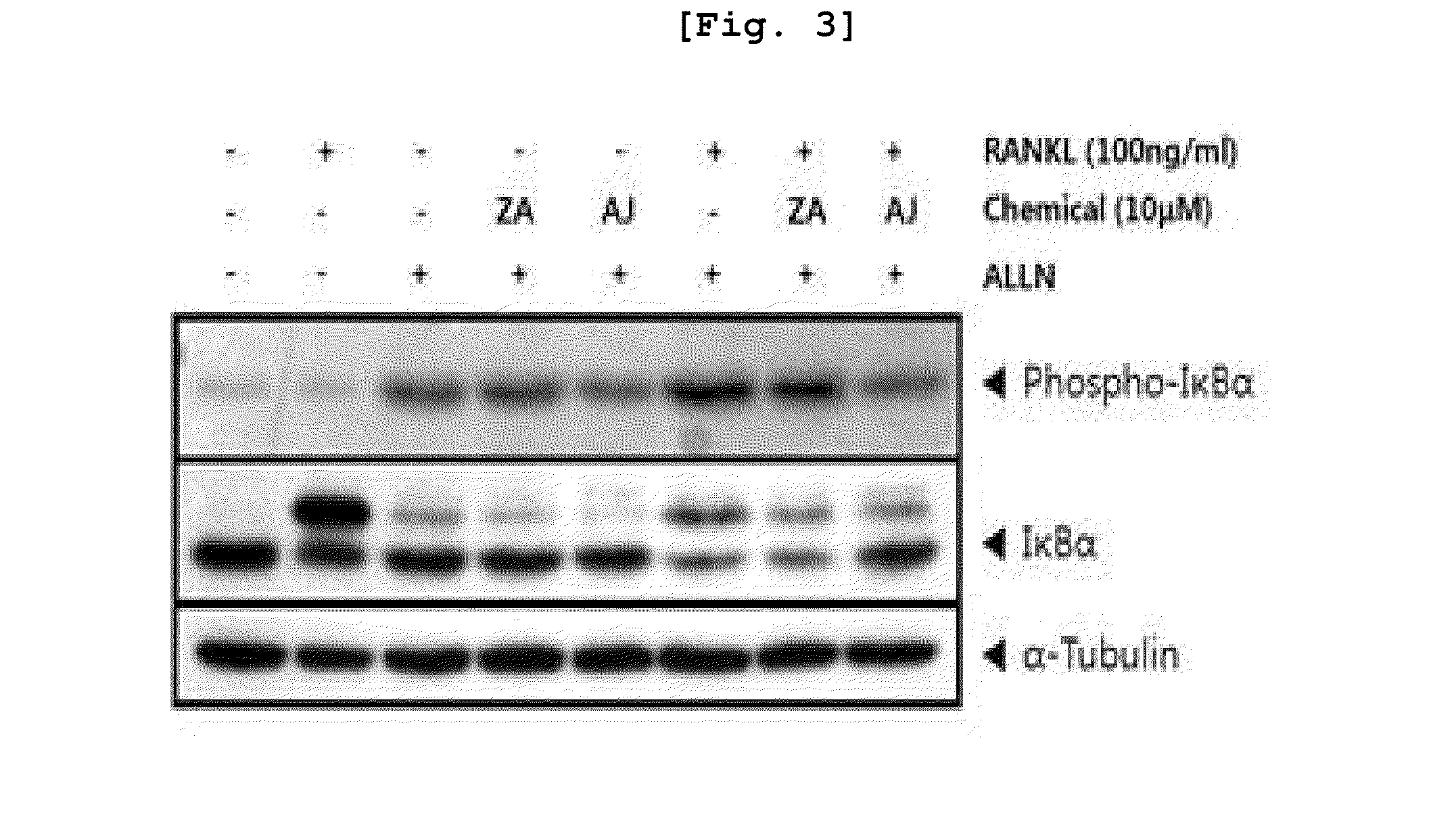
New compounds for inhibiting differentiation of osteoclast and pharmaceutical composition comprising thereof
a technology of osteoclast differentiation and new compounds, which is applied in the direction of drug compositions, heterocyclic compound active ingredients, organic chemistry, etc., can solve the problems of cancer patients' death, cancer, and uncertain cause of cancer, and achieve inhibitory effect on osteoclast differentiation and inhibitory effect on nf-b transcription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Preparation of Compound 4
[0201]
TABLE 1Substituents of Compound 4NoRNoRa2,6-diisopropylphenylbphenylc2-chlorophenyld3-chlorophenyle4-chorophenylf2-trifluoropentylphenylgcyclohexylh4-carboxy-3-hydroxyphenyli3-caboxy-4-hydroxyphenylj2-fluorophenylK3-fluorophenyll4-fluorophenylmbenzyln2-fluorobenzylo3-fluorobenzylp4-fluorobenzylqphenethylr2-fluorophenethyls3-fluorophenethylt4-fluorophenethyl
(1) 3,4-diphenylfuran-2,5-dione (3)
[0202]After dissolving compound 2 (19.6 g, 96.0 mmol) in acetic anhydride (250 ml), Compound 1 (18.0 g, 80.0 mmol) is added thereto. After 6 hours of reaction with reflux, the temperature was reduced to room temperature, three times extractions were carried out with EA and seven times washings were carried out with brine. Water was removed with anhydrous sodium sulfate and to remove the remaining phenyl acetic acid, the column chromatography was carried out with EA / HX (1 / 2). After the distillation under the reduced pressure for the removal of the solvents conduc...
example 2
The preparation of Compounds 5, 6 and 7
[0225]
(1) The synthesis of N,2-diphenylacetamide (5a)
[0226]After dissolving compound 2 (1.00 g, 7.34 mmol) in MC (30 ml), the activation for 30 minutes was carried out by the addition of DCC (1.82 g, 8.81 mmol). After the sufficient activation, aniline (0.75 g, 8.07 mmol) was added thereto and the agitation for 6 hours was carried out at room temperature. After the identification of the reaction progress with TLC, DCU was removed by the filtration and solvents were removed by the distillation under the reduced pressure. Then, the next reaction was followed without the purification process.
(2) The synthesis of N-(2-chlorophenyl)-2-phenylacetamide (5b)
[0227]After dissolving compound 2 (5.00 g, 36.7 mmol) in MC (50 ml), the activation for 30 minutes was carried out by the addition of DCC (9.10 g, 44.5 mmol). After the sufficient activation, 2-chloroaniline (5.15 g, 40.4 mmol) was added thereto and the agitation for 6 hours was carried out at room ...
example 3
The Preparation of Compound 8
[0242]
[0243]The synthesis of 5-hydroxy-1,3,4-triphenyl-1H-pyrrol-2(5H)-one(8)
[0244]After the addition of compound 4e (1.00 g, 2.78 mmol) in methanol, NaBH4 was slowly added thereto in acetone-ice bath and the agitation for 3 hours was carried out at room temperature. After the identification of disappearance of compound 4e with TLC, quenching was carried out with in HCL aqueous solution in the ice bath. Then, three times extractions were carried out with EA and three times washings were carried out with brine. Water was removed by using Na2SO4, solvents were removed and then, the column chromatography was carried out with EA / HX (1 / 4). After the distillation under the reduced pressure, vacuum drying was carried out to obtain compound 8
[0245]white solid (0.71 g, 70.9%); mp 186-187° C.; IR (KBr, cm−1) 3326, 1672; 1H NMR (CDCl3-d) δ 6.325-6.503 (m, 1H), 7.279-7.476 (m, 12H), 7.847-7.869 (m, 2H); 13C NMR (CDCl3-d) δ 82.205, 121.498, 127.793, 127.884, 128.043,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com