Self-Assembling Peptide and Peptide Gel with High Strength
a peptide gel and self-assembling technology, applied in the direction of peptide/protein ingredients, spectral modifiers, macromolecular non-active ingredients, etc., can solve the problems of insufficient transparency of gel, scaffold may collapse when grasped with tweezers, and unknown infectious diseases, etc., to achieve the effect of practical mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
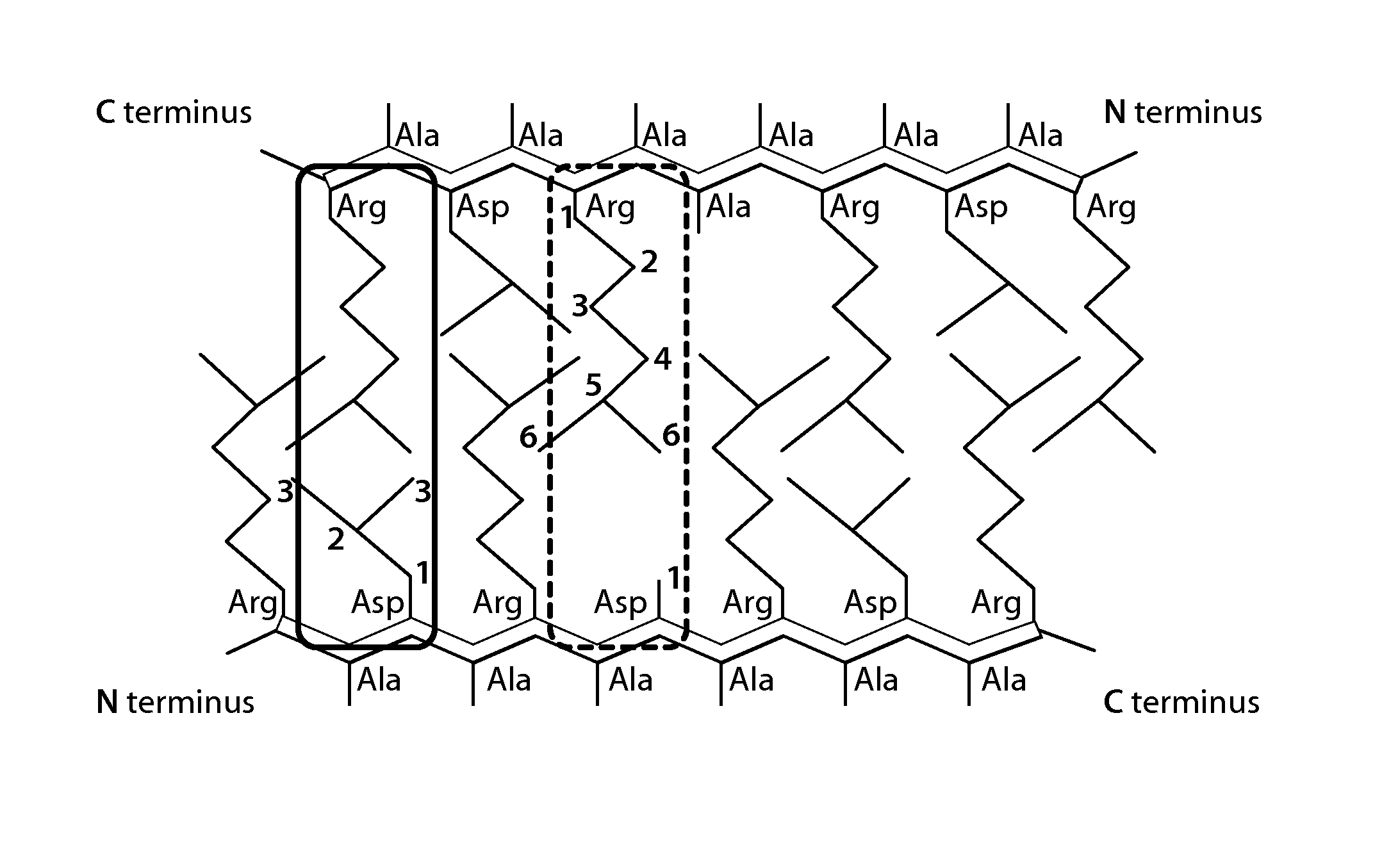
[0101]A self-assembling peptide formed of an amino acid sequence of SEQ ID NO: 1 described in Table 1 was synthesized by an Fmoc solid-phase synthesis method. Next, the self-assembling peptide was acetylated at the N-terminus and amidated at the C-terminus by a conventional method to afford a modified peptide 1 ([CH3CO]-RLDLRLALRLDLR-[NH2]).
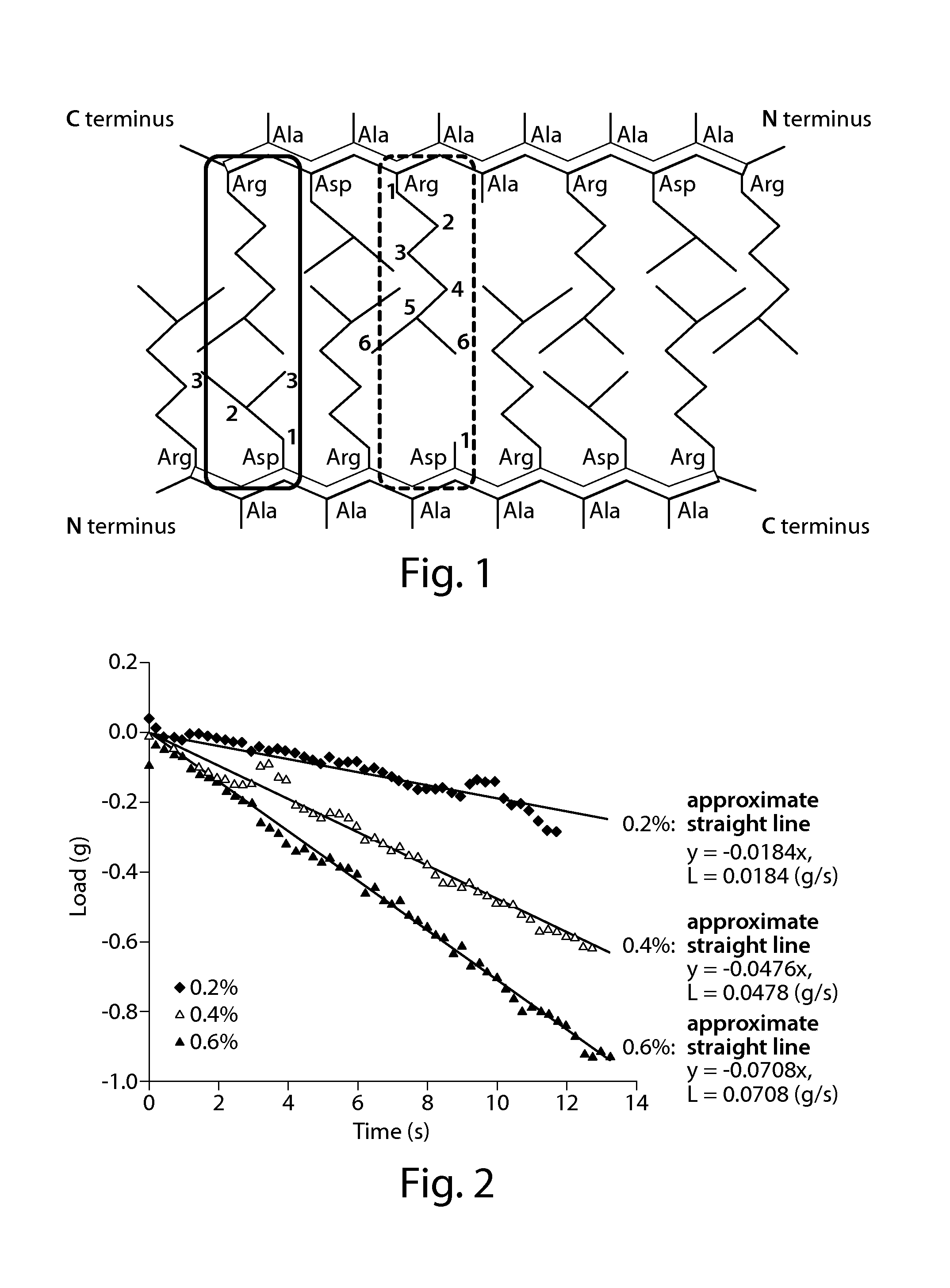
[0102]The resultant modified peptide 1 was dissolved in a 0.1 wt % sodium hydrogen carbonate solution at concentrations of 0.2, 0.4, and 0.6 w / v % to afford peptide solutions. The resultant peptide solutions were each measured for a pH value using pH test paper (trade name: pH Indicator Papers, manufactured by Whatman International Ltd., measurement range pH=6.0 to 8.1, Cat. No. 2629 990), and the pH was found to fall within the range of 6.9 to 7.8. 300 μl each of the peptide solutions were charged into a trade name “Nunc Tissue Culture Inserts” (membrane diameter: 10 mm, pore size: 8.0 μm membrane material: polycarbonate) (product number “Cat. N...
example 2
[0107]A modified peptide 2 ([CH3CO]-RLDLRLLLRLDLRe [NH2]) was obtained in the same manner as in Example 1 except that an amino acid sequence of SEQ ID NO: 2 was adopted in place of the amino acid sequence of SEQ ID NO: 1. Peptide gels 2 (liquid phase: MEN) at peptide concentrations of 0.2, 0.4, and 0.6 w / v % were formed in the same manner as in Example 1 except that the modified peptide 2 was used in place of the modified peptide 1.
[0108]The resultant peptide gels were each measured for mechanical strength in the same manner as in Example 1. FIG. 4 shows the results. As shown in FIG. 4, the peptide gel 2 at 0.4 w / v % had an absolute value L of an amount of change in load per unit time of 0.0423 g / s in the compression test.
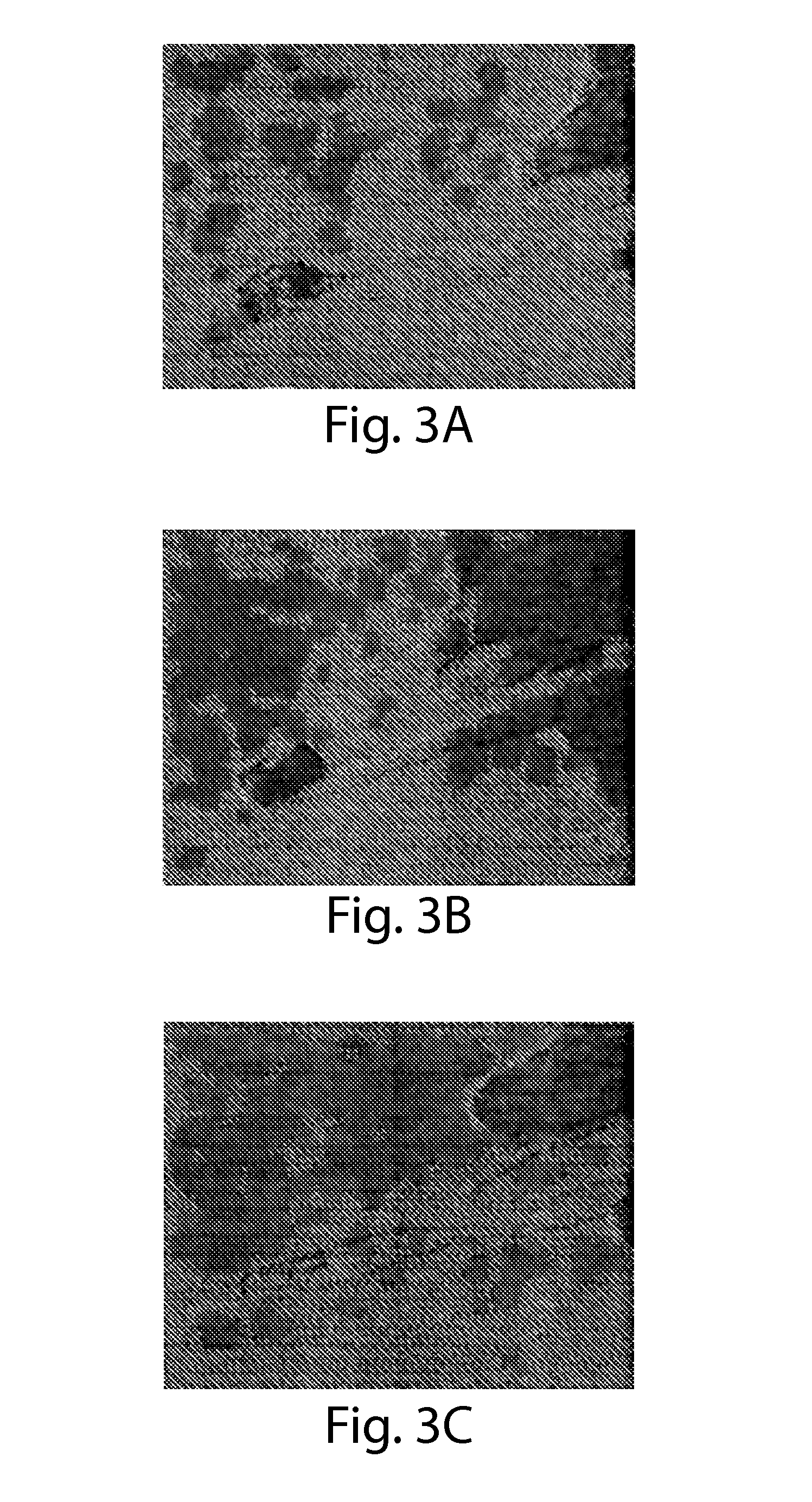
[0109]The peptide gel 2 at 0.4 w / v % was grasped with tweezers and transported. As a result, as shown in FIG. 3(b), the peptide gel 2 had sufficient strength to grasp and was excellent in handleability.
example 3
[0110]A modified peptide 3 ([CH3CO]-RLDLRLALRLDLRL-[NH2]) was obtained in the same manner as in Example 1 except that an amino acid sequence of SEQ ID NO: 3 was adopted in place of the amino acid sequence of SEQ ID NO: 1. Peptide gels 3 (liquid phase: DMEM) at peptide concentrations of 0.2 and 0.4 w / v % were formed in the same manner as in Example 1 except that the modified peptide 3 was used in place of the modified peptide 1.
[0111]The resultant peptide gels were each measured for mechanical strength in the same manner as in Example 1. FIG. 5 shows the results. As shown in FIG. 5, the peptide gel 3 at 0.4 w / v % had an absolute value L of an amount of change in load per unit time of 0.0336 g / s in the compression test.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com