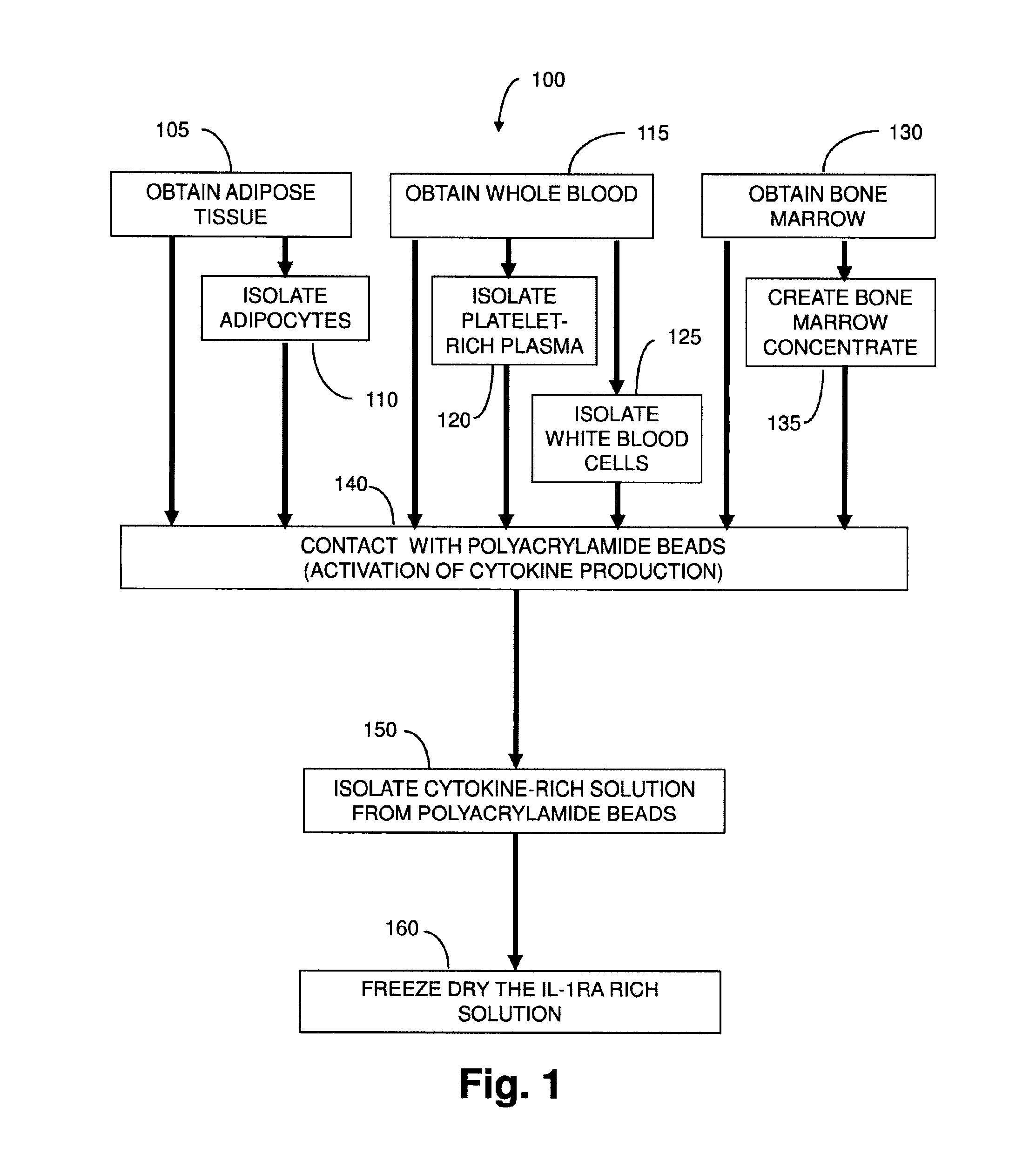
Treatment of collagen defects using protein solutions
a technology of collagen and protein solution, which is applied in the direction of peptide/protein ingredients, peptide sources, drug compositions, etc., can solve the problems of genetic defects or nutritional deficiencies, wear, trauma or disease, and may be debilitating and painful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparing and Characterizing a Protein Solution
[0163]A Protein Solution rich in interleukin-1 receptor antagonist is prepared from seven consented human providers. Blood (55 mL) is drawn into a 60 cc syringe with 5 mL of anticoagulant citrate dextrose solution A (ACD-A, Citra Labs, Braintree, Mass.). Platelet-rich plasma (PRP) is created using the GPS® III platelet concentration system (800-1 003A, Biomet Biologics, Warsaw, Ind.) according to the instructions for use. The solution is generated by adding 6 mL of PRP to a modified Plasmax device containing 1 gram of polyacrylamide beads (Biomet Biologics, Warsaw, Ind.). The IL-Ira solution is removed from the Plasmax devices and frozen at minus 50° C. for the assay. Cytokine content is assayed on a 16-plex ELISA (Searchlight Protein Array, Aushon Biosystems, Billerica, Mass.). The analytes included IL-4, IL-10, IL-11, IL-13, IL-Ira, IFN-γ, sTNF-RI, sTNF-RII, IL-1α, IL-1β, TNF-α, IL-17, IL-18, bFGF, TBF-β1, and TBF-β2.
[0164]The solutio...
example 2
Generation of IL-1ra from Platelet-Rich Plasma
[0165]An IL-1ra-rich solution is created as follows. Whole blood (70 mL) anticoagulated (10%) with ACD-A (Braintree, Mass., USA) is drawn from 5 healthy volunteers. A portion (10 mL) is reserved for a whole blood measurement. Platelet-rich plasma (PRP) (6 mL) is produced using the GPS® II System (Biomet Biologics, LLC, Warsaw, Ind., USA). Complete blood counts are collected for the whole blood and PRP samples following a validated procedure, as described in Woodell-May J E, Ridderman D N, Swift M J, Higgins J. “Producing Accurate Platelet Counts for Platelet Rich Plasma: Validation of a Hematology Analyzer and Preparation Techniques for Counting” J. Craniofac. Surg. (2005) September. 16(5):749-56.
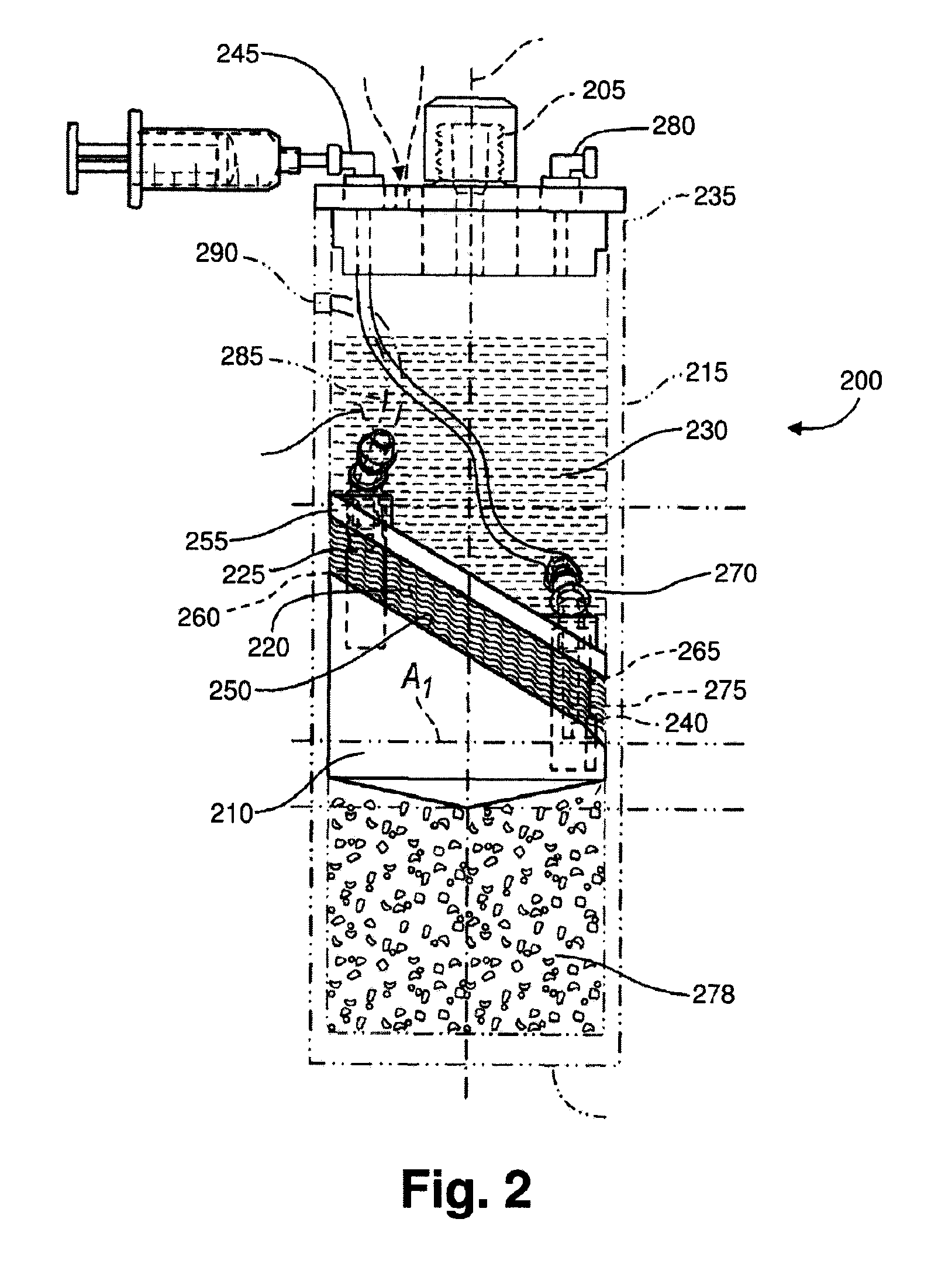
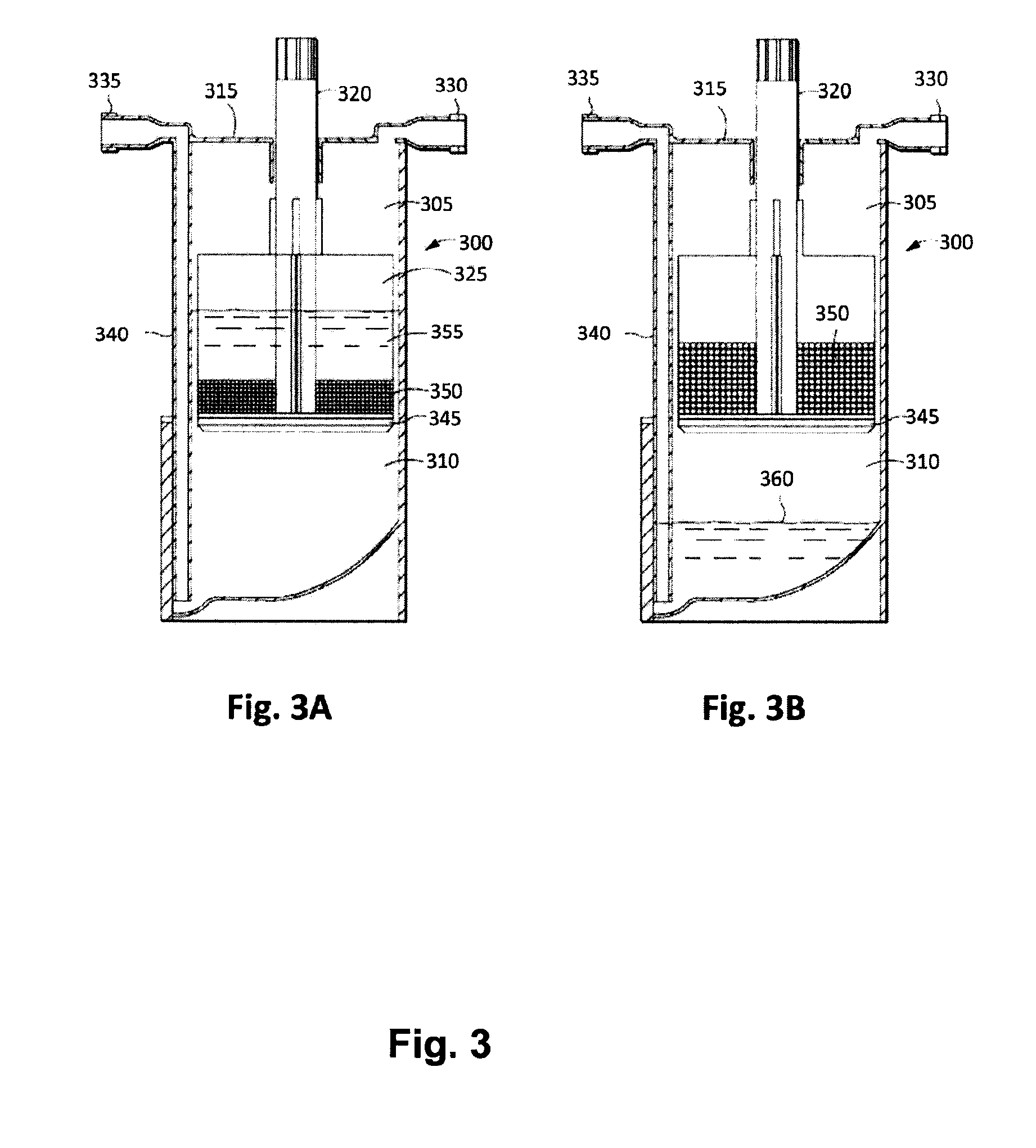
[0166]Following the PRP production, 5 mL of the PRP is added to a modified plasma concentration device (Plasmax™, Biomet Biologics LLC, Warsaw, Ind., USA) and incubated with polyacrylamide desiccating beads in the device for 24 hours at room tem...
example 3
Production of Protein Solution from PRP
[0170]Anticoagulated blood (120 cc) is collected from 5 human donors. Platelet-rich plasma (PRP) is prepared using GPS® III disposables (Biomet Biologics LLC, Warsaw, Ind., USA). PRP is loaded into modified plasma concentration devices (Plasmax™, Biomet Biologics LLC, Warsaw, Ind., USA) and processed. The output is divided into 4 groups: IL-1ra in concentrated plasma with and without thrombin activation (1000 U / mL in 1M CaCl2), or cell-free IL-1ra with and without thrombin activation. IL-1ra is measured using ELISA (R&D Systems) over time.
[0171]The PRP contacts polyacrylamide beads in the Plasmax™ device while electromagnetic field stimulation is provided using a capacitively coupled electromagnetic field.
[0172]Unclotted PRP produces an average of about 50 ng over 24 hrs. The cell-free samples produce about 34 ng without changing over 24 hrs. Once clotted, the elution of IL-1ra is slowed, with only about 30% being eluted after 10 hours. Release...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com