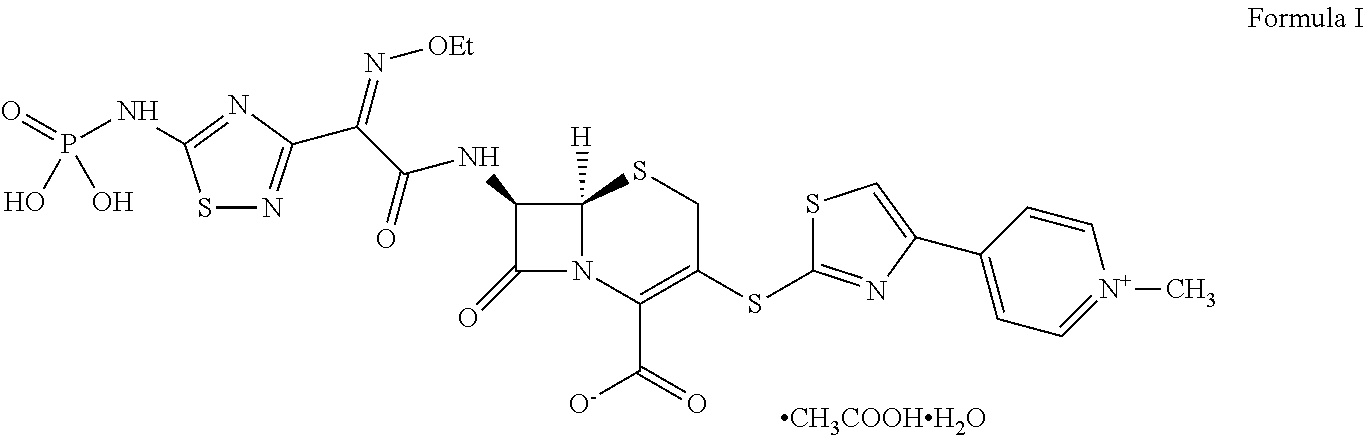
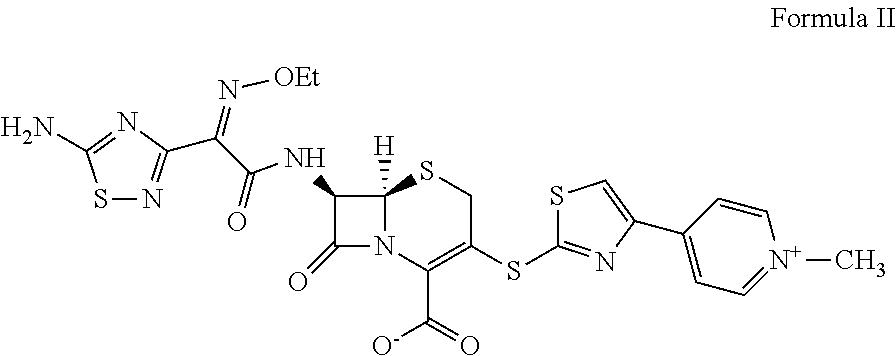
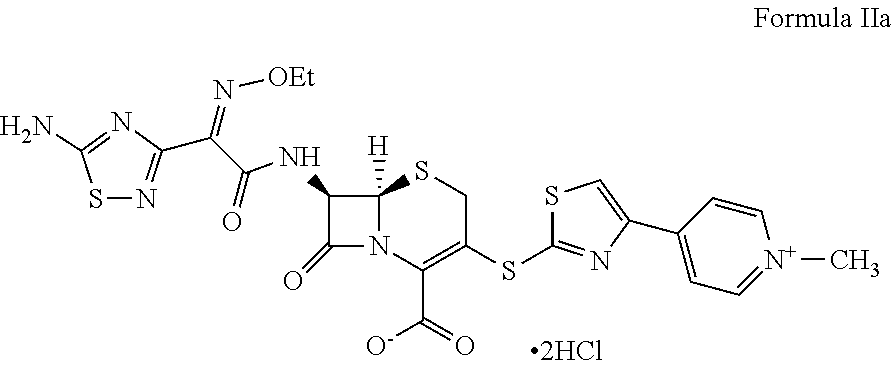
Process for preparing ceftaroline salts or hydrates thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of S-1,3-Benzothiazol-2-Y1(2z)-(5-Amino-1,2,4-Thiadiazol-3-Y1)(Ethoxyimino)Ethanethioate (Reactive Derivative of Formula IV)
[0046]Triphenylphosphine (16 g, 0.06 moles) and (2Z)-(5-amino-1,2,4-thiadiazol-3-yl)(ethoxyimino)ethanoic acid (Formula IV; 10 g, 0.046 moles) were treated in dichloromethane (60 mL) at 25° C. to 30° C. The resulting reaction mass was stirred for 1 to 2 hours at 25° C. to 30° C. and then cooled to 0° C. 2,2′-Disulfanediylbis(1,3-benzothiazole) (20.2 g, 0.06 moles) was added to the reaction mass at 0° C. to 5° C. Triethylamine (5.14 g, 0.051 moles) was added drop wise to the reaction mass over 5 to 10 minutes at 0° C. to 5° C. The resulting reaction mass was stirred for 3 to 4 hours at 0° C. to 5° C. and was allowed to stand for 1 to 4 hours. The solid obtained was filtered, washed with dichloromethane, and dried in an air oven for 10 to 12 hours at 40° C. to 45° C. Yield=22.2 g (88% yield)
example 2
Preparation of Ceftaroline Dihydrochloride (Formula IIa)
[0047]7-Amino-3-{[4-(1-methyl pyridinium-4-yl)-1,3-thiazol-2-yl]sulfanyl}-8-oxo-5-thia-1l-aza bicyclo[4.2.0]oct-2-ene-2-carboxylate hydrochloride (hydrochloride salt of Formula III; 10 g, 0.0208 moles) and S-1,3-benzothiazol-2-yl(2Z)-(5-amino-1,2,4-thiadiazol-3-yl)(ethoxyimino)ethanethioate (reactive derivative of Formula IV; 22.2 g, 0.042 moles) were treated in a mixture of tetrahydrofuran and water (3:2; 200 mL) at 25° C. to 30° C. Tributylamine (10.7 g, 0.0581 moles) was added in 10 minutes and the resulting mass was stirred for 3 to 5 hours at 30° C. to 35° C. Ethyl acetate (100 mL) was added to the resulting mass. It was stirred and the aqueous layer was separated. Ethanol (150 mL) and hydrochloric acid (35%; 30 mL) were added to the aqueous layer at 15° C. to 20° C. and the resulting mass was stirred for 10 to 12 hours at 15° C. to 20° C. The solid obtained was filtered, washed with ethanol, and dried under vacuum for 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com