Producing lithium
a lithium-ion-coated composite layer and lithium-ion-coating technology, which is applied in the direction of electrolysis components, instruments, optics, etc., can solve the problems of high reactive and flammable lithium, damage to alkali metal ion-coatings, and previous lithium-producing systems have involved substantial capital and operating costs, and achieves reasonable energy-consuming and stable lithium-ion-coating composite layers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
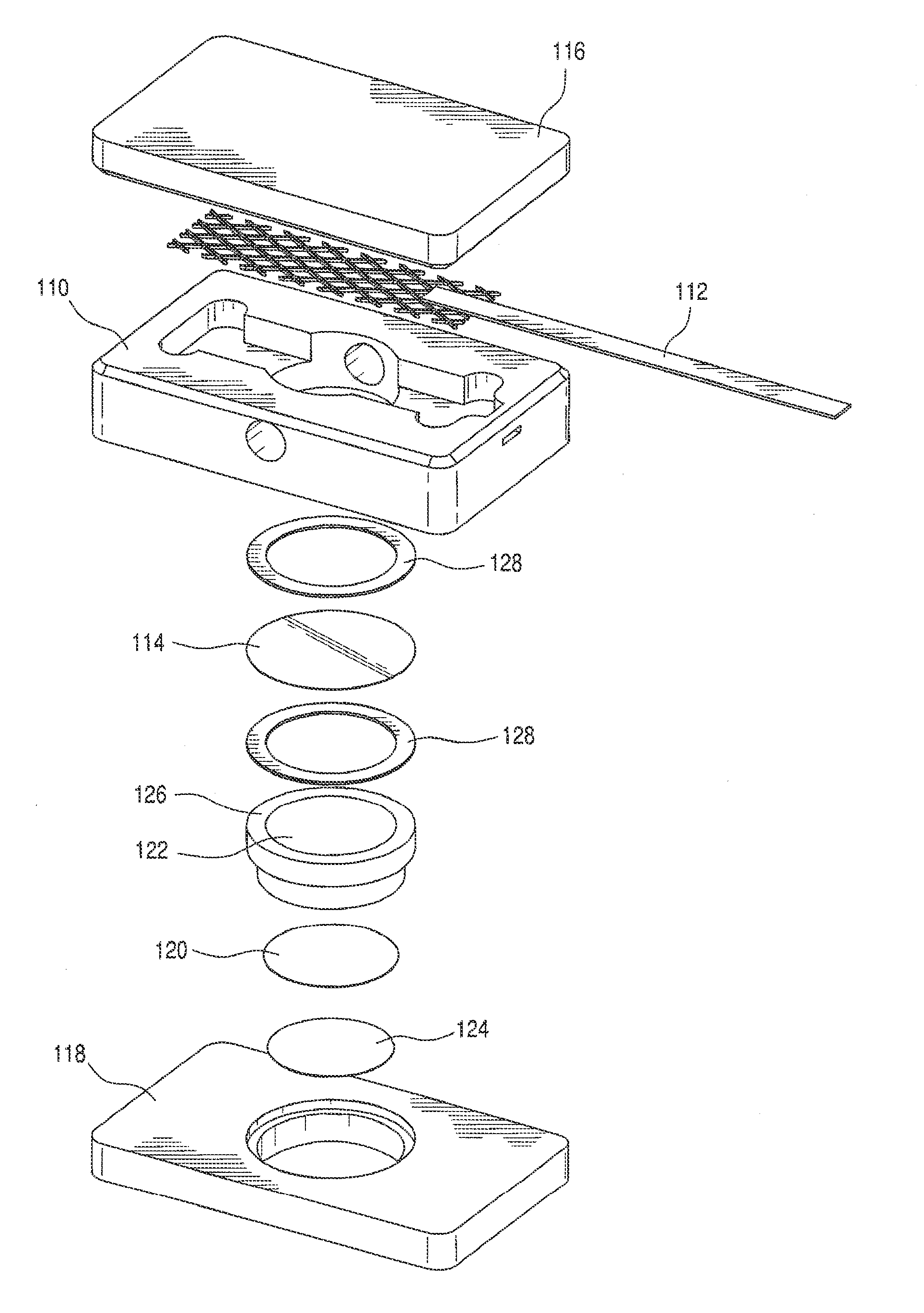
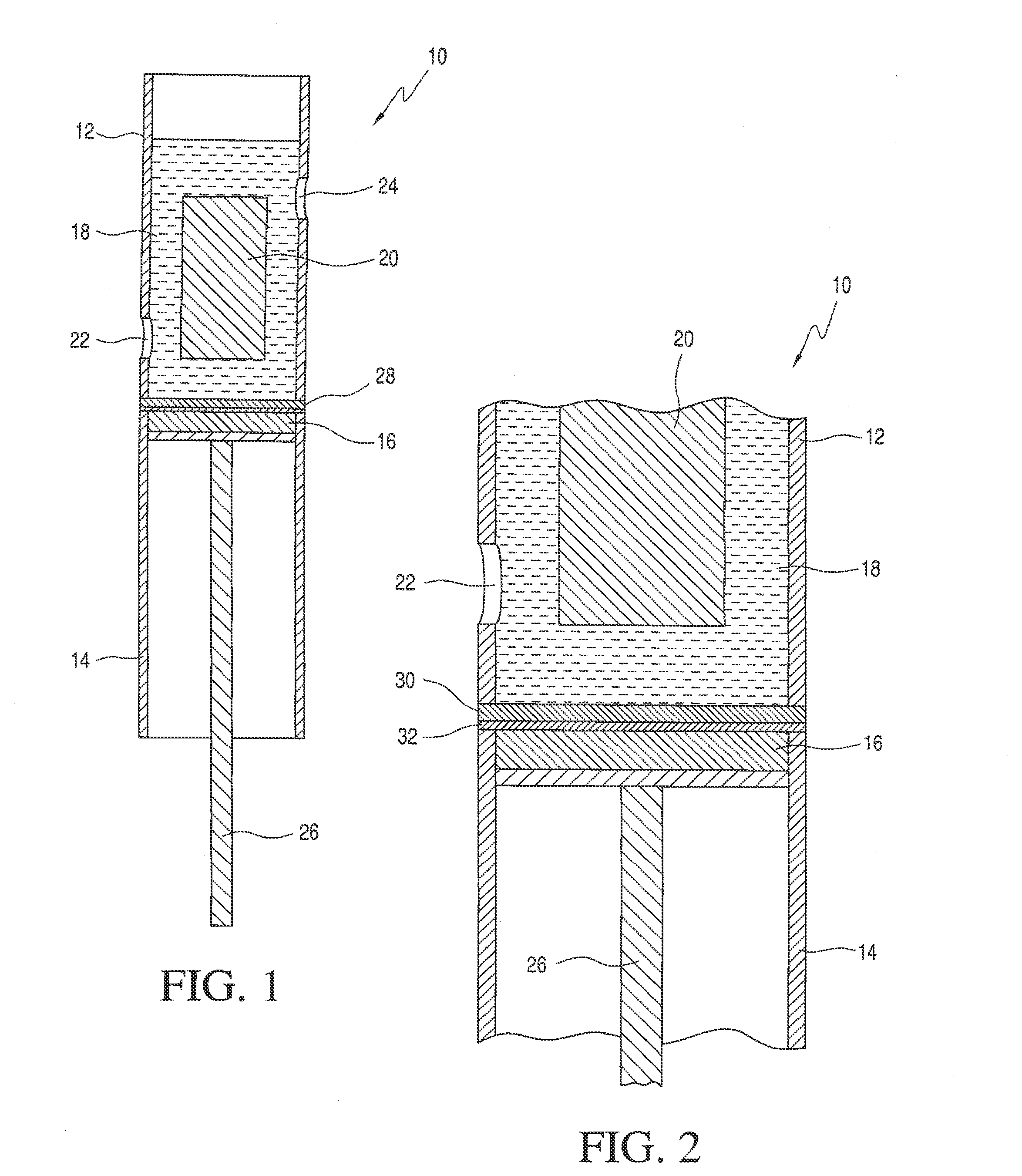
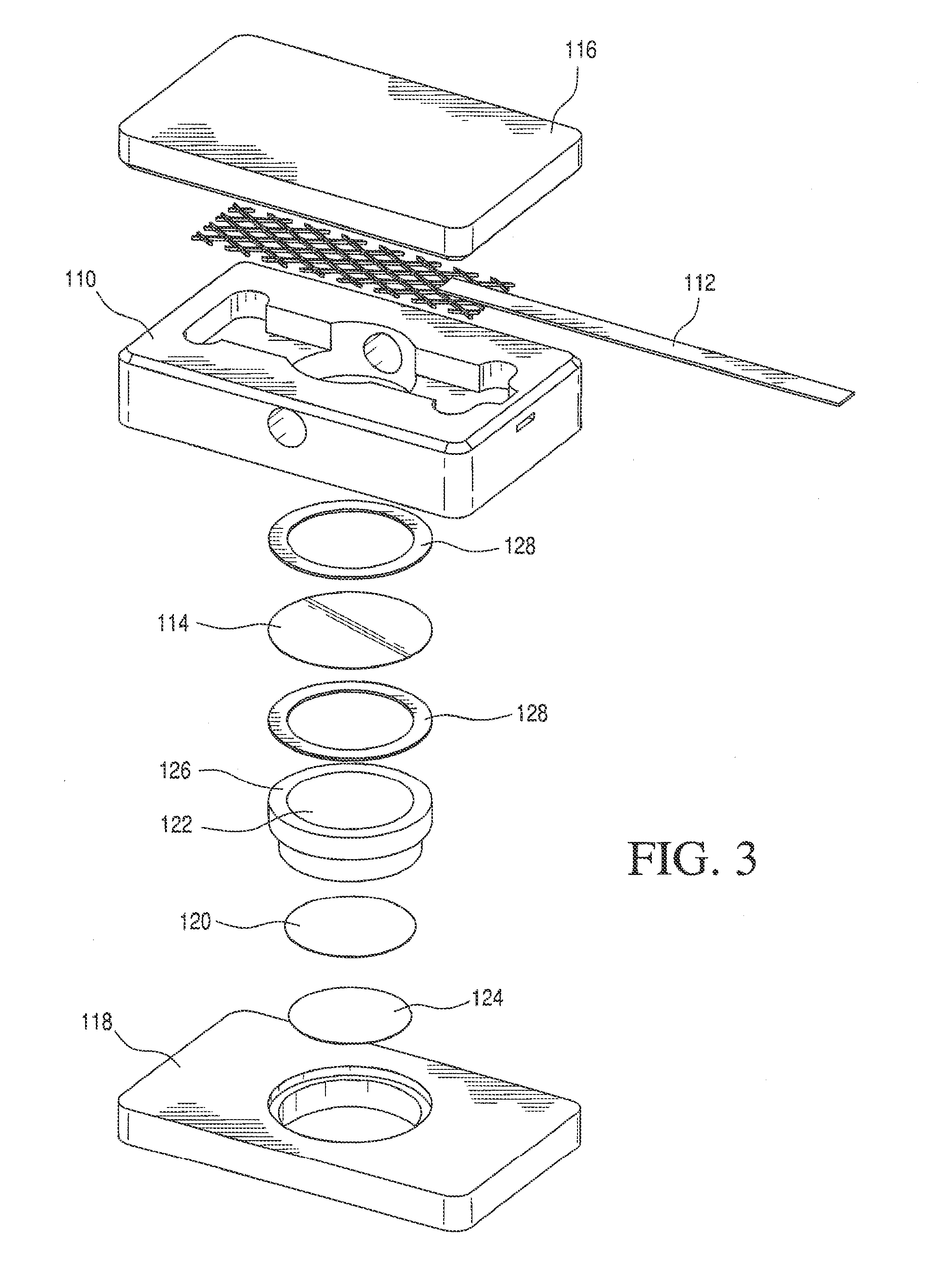
[0036]The cell used is shown schematically in FIG. 3. The cell 110 includes cell cover 116, retainer 118, Pt anode 112, cathode 124 and a LI-GC conductive glass 114 with lithium ion conductive barrier film 120 incorporated into a porous polyolefin flat-film membrane 122. The supported LI-GC-BF multilayer is intercalated between cathode 124 and a lithium ion-rich electrolyte 18 (in FIGS. 1 and 2). The cell further comprises supporting Teflon® sleeve structure 126 with gaskets 128. One gasket seals between the LI-GC and the housing to prevent leakage of the electrolyte from the anode compartment into the cathode compartment. The other gasket allows for even compression of the LI-GC by the Teflon Sleeve to prevent breakage of the LI-GC plate.
[0037]The cell 110 includes anode 112 that is a platinized titanium anode, 1″×4″ rhodium and palladium jewelry plating). The cathode is a palladium cathode disk fabricated in-house, 1.4 inch round. The LI-GC 114 material is LICGC® G71-3 N33: DIA 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic conductivity | aaaaa | aaaaa |
| lithium ion conductive | aaaaa | aaaaa |
| ion conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com