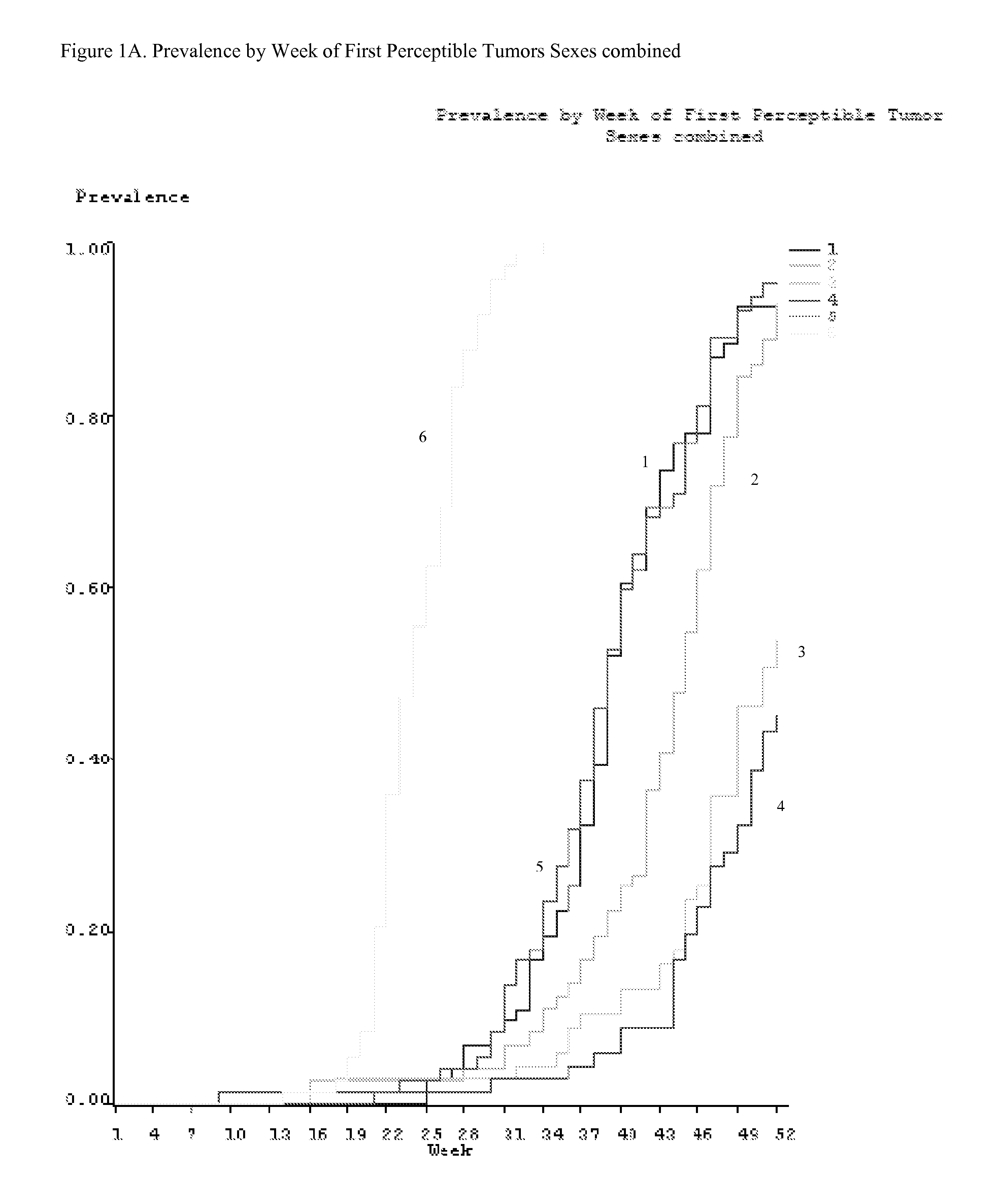
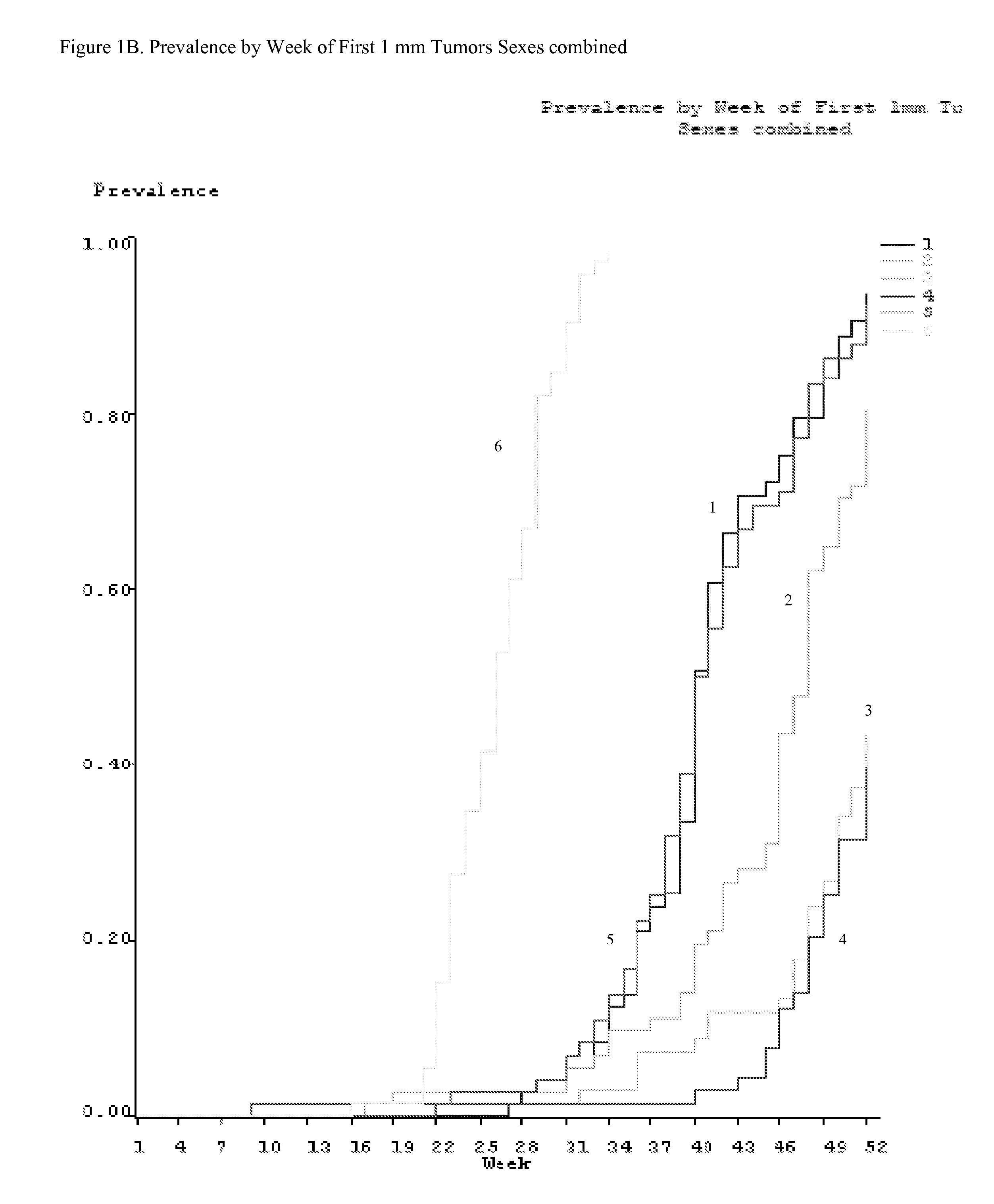
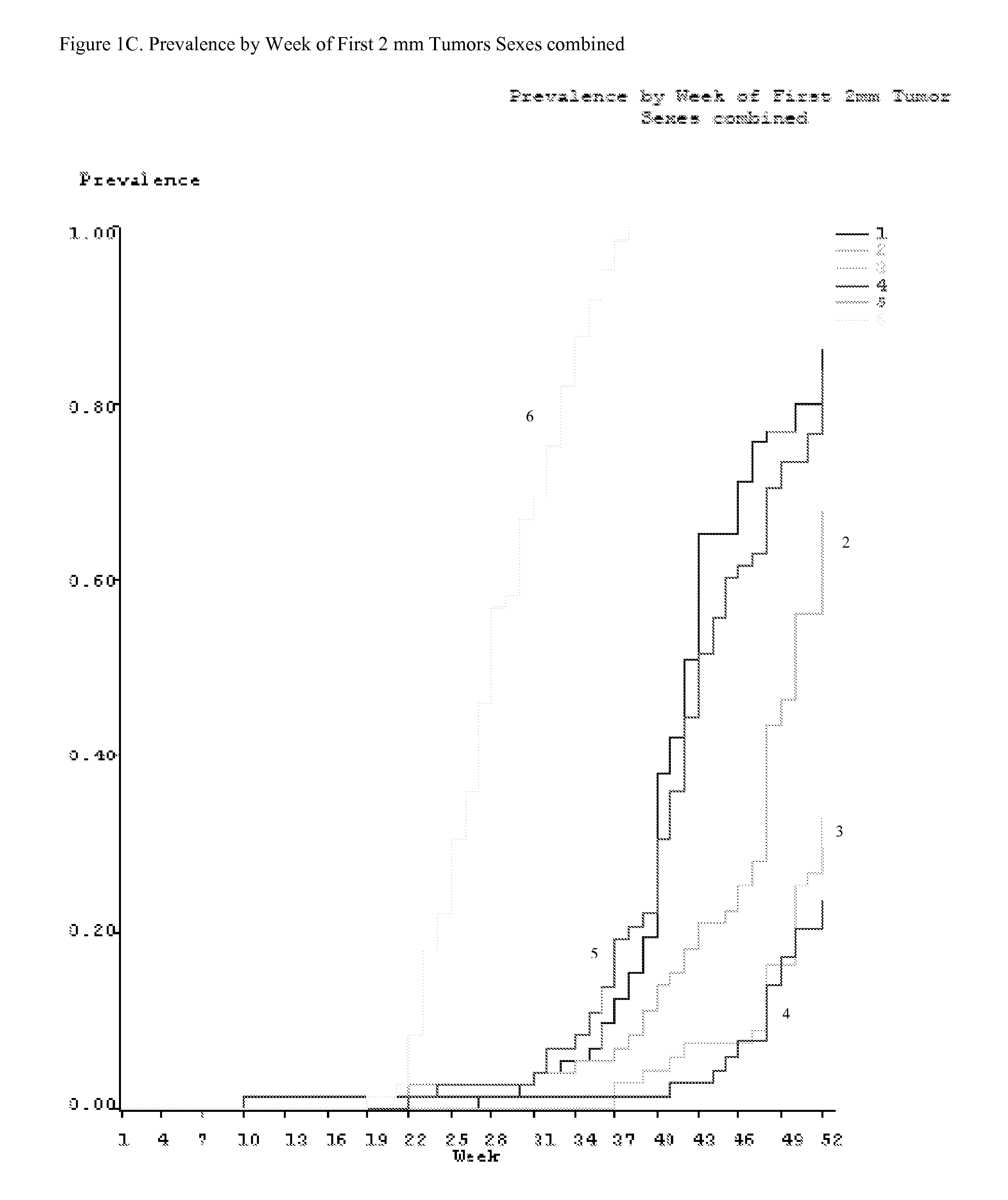
Method for preventing or treating skin tumor
a skin tumor and adrenergic receptor technology, applied in the field of skin tumor prevention or treatment, can solve the problems of significant regression in skin tumor growth in subjects exposed to uv radiation, significant delay in skin tumor formation, etc., and achieve the effect of safe and effective amount of 2 adrenergic receptor agonis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Aqueous Topical Formulations
[0099]This example illustrates aqueous topical formulations that can be used in the present invention.
[0100]A first aqueous solution topical formulation comprises: brimonidine tartrate (0.01% to 5% w / w); Puriteg (0.005% w / w) (stabilized chlorine dioxide) as a preservative; and the inactive ingredients: boric acid; calcium chloride; magnesium chloride; potassium chloride; purified water; sodium borate; sodium carboxymethylcellulose; sodium chloride; with hydrochloric acid and / or sodium hydroxide to adjust the pH to 5.6 to 6.6. The osmolality is in the range of 250-350 mOsmol / kg.
[0101]A second aqueous solution topical formulation comprises brimonidine tartrate (0.2% to 2% w / w); benzalkonium chloride (0.005% w / w.) as a preservative; and the inactive ingredients: boric acid; calcium chloride; magnesium chloride; potassium chloride; purified water; sodium borate; sodium carboxymethylcellulose; sodium chloride; with hydrochloric acid and / or sodium hydroxide to ...
example 2
Cream or Ointment Topical Formulations
[0102]This example illustrates cream or ointment topical formulations that can be used in the present invention.
[0103]A first cream topical formulation (hydrophilic ointment) is described in Table 2 below.
TABLE 2IngredientWeight PercentBrimonidine tartrate0.01% to 5%Stearic acid 7%Stearyl alcohol5% Cetyl alcohol2% Glycerin 10%Sodium lauryl sulfate 1%Propylparaben0.05%Methylparaben0.25%Disodium edetate0.055% Distilled waterQSTOTAL 100%
[0104]To make the formulation, the stearyl alcohol and the white petrolatum are melted on a steam bath, and warmed to about 75 degrees C. The other ingredients, previously dissolved in the water and warmed to 75 degrees C., are then added, and the mixture is stirred until it congeals. The mixture is then allowed to cool with stirring, and brimonidine tartrate is then added as a concentrated solution.
[0105]An ointment topical formulation (hydrophilic ointment) is described in Table 3 below.
TABLE 3IngredientsWeight...
example 3
Gel Topical Formulations
[0107]This example illustrates gel topical formulations that can be used in the present invention.
[0108]A first gel formulation is described in Table 4 below.
TABLE 4IngredientsWeight %Brimonidine tartrate0.01-5% Methylparaben NF0.15%Propylparaben NF0.03%Hydroxyethylcellulose NF1.25%Disodium Edetate USP0.05%Purified Water, USPQSTOTAL 100%
[0109]A second gel formulation is described in Table 5 below.
TABLE 5IngredientsWeight %Brimonidine tartrate 0.5%Methylparaben0.20%Propylparaben0.05%Carbomer 934P NF 1.0%Sodium HydroxideQS pH 7Purified Water, USPQSTOTAL 100%
[0110]The ingredients are mixed together and aqueous sodium hydroxide is slowly added to the mixture until a pH of about 7 is reached and the gel is formed.
[0111]A third gel formulation is described in Table 6 below.
TABLE 6IngredientWeight PercentBrimonidine tartrate0.1-2% Carbomer 934P1.25% Methylparaben0.3%Phenoxyethanol0.4%Glycerin5.5%10% Titanium dioxide0.625% Propylene glycol5.5%10% NaOH Solution6.5%D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com