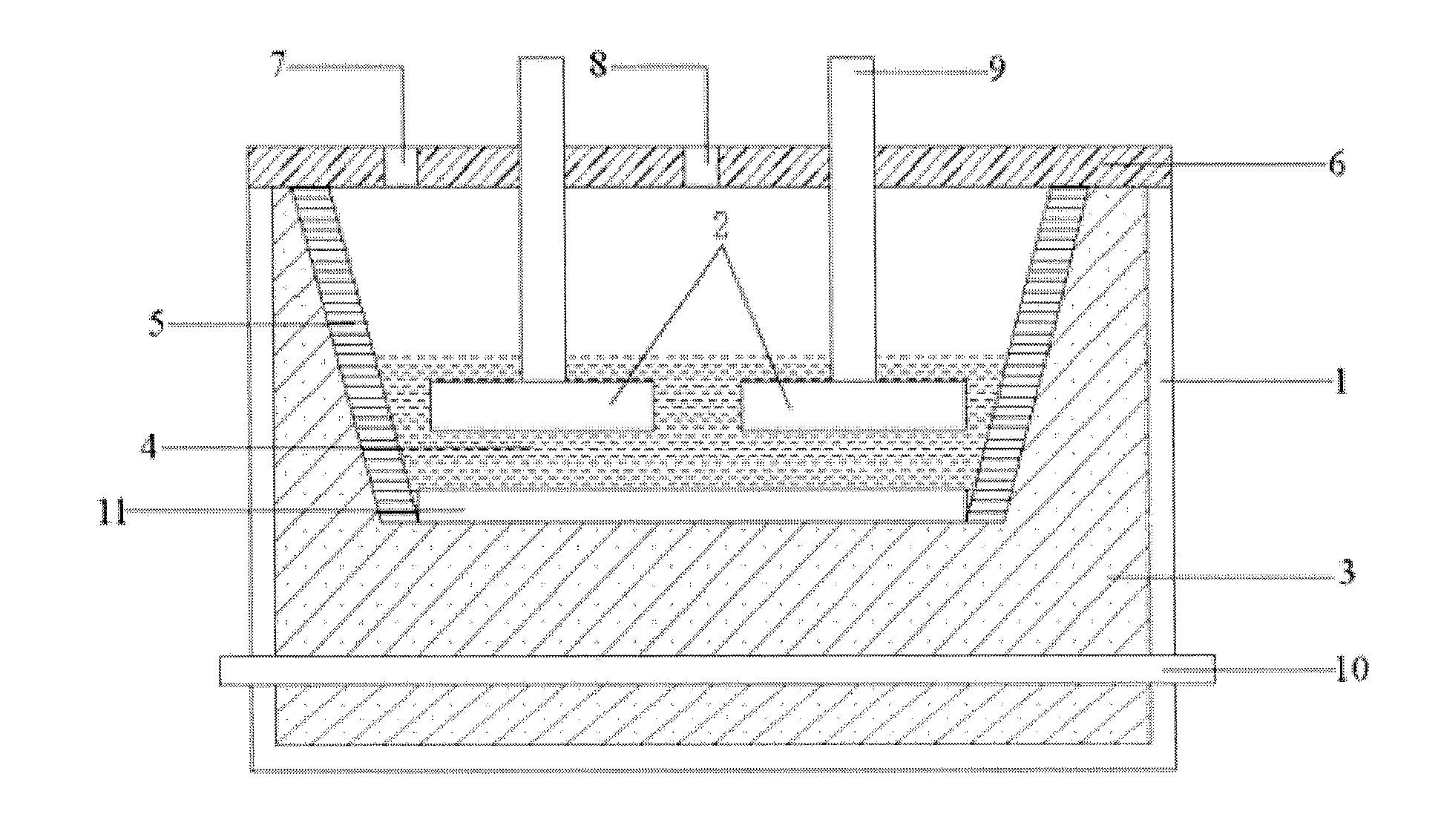
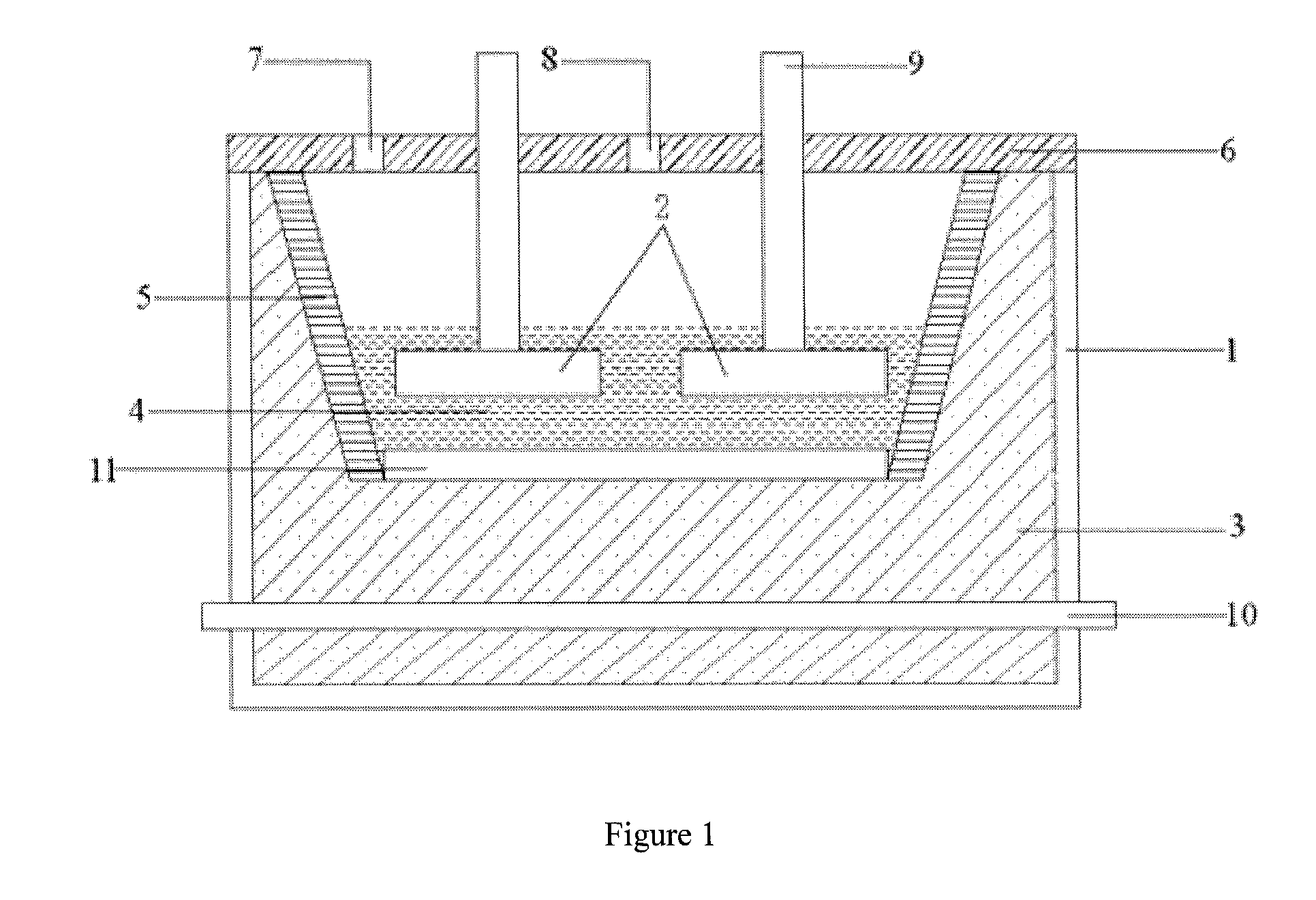
Electrolysis tank used for aluminum electrolysis and electrolysis process using the electrolyzer
a technology of electrolysis cell and electrolysis process, which is applied in the direction of chemistry apparatus and processes, separation processes, and dispersed particle separation, etc., can solve the problems of low power consumption of aluminum electrolysis process, low electrical conductivity, and increase in alloy anode cost, etc., to achieve low power consumption in aluminum electrolysis process, low overvoltage, and high electric conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0042]Fe, Cu, Ni and Sn metal blocks are mixed based on 23 wt % of Fe, 60 wt % of Cu, 14 wt % of Ni and 3 wt % of Sn, the mixture is molten by heating at high temperature and then subjected to casting to obtain an anode 1. The anode 1 has a density of 8.3 / cm3, a specific resistivity of 68 μΩ·cm and a melting point of 1360° C.
[0043]The components of the electrolyte in this embodiment are as follows: 32% of NaF, 57% of AlF3, 3% of LiF, 4% of KF and 4% of Al2O3, wherein the molar ratio of NaF to aluminum fluoride AlF3 is 1.12. The liquidus temperature of the electrolyte in this embodiment is 640° C. according to measurement. The electrolyte has an electric conductivity of about 1.7 Ω1·cm−1, a density of about 2.03 g / cm3 and an alumina saturation concentration of 5%.
[0044]The process using the electrolyte in the present invention for aluminum electrolysis is as follows:
(1) by means of the anode 1 and the carbon body cathode, melting the aforementioned amounts of NaF, AlF3, LiF, KF and A...
embodiment 2
[0046]Fe, Cu, Ni and Sn metal blocks are mixed based on 40 wt % of Fe, 36 wt % of Cu, 19 wt % of Ni and 5 wt % of Sn, the mixture is molten by heating at high temperature and then subjected to casting to obtain an anode 2. The anode has a density of 8.1 / cm3, a specific resistivity of 76.8 μΩ·cm and a melting point of 1386° C.
[0047]The components of the electrolyte in this embodiment are as follows: 38% of NaF, 50% of AlF3, 2% of LiF, 5% of KF and 5% of Al2O3, wherein the molar ratio of NaF to aluminum fluoride AlF3 is 1.52.
[0048]The performances of the electrolyte in this embodiment are measured and the measurement result is that the liquidus temperature of the electrolyte in this embodiment is 670° C. The electrolyte has an electric conductivity of about 1.8 Ω−1·cm−1, a density of about 2.05 g / cm3 and an alumina saturation concentration of 6%.
[0049]The process using the electrolyte in the present invention for aluminum electrolysis is as follows:
(1) by means of the anode 2 and the ...
embodiment 3
[0051]Fe, Cu, Ni and Sn metal blocks are mixed based on 25 wt % of Fe, 46.8 wt % of Cu, 28 wt % of Ni and 0.2 wt % of Sn, the mixture is molten by heating at high temperature and then subjected to casting to obtain an anode 3. The anode has a density of 8.2 / cm3, a specific resistivity of 72 μΩ·cm and a melting point of 1350° C.
[0052]The components of the electrolyte in this embodiment are as follows: 32% of NaF, 57% of AlF3, 3% of LiF, 4% of KF and 4% of Al2O3, wherein the molar ratio of NaF to aluminum fluoride AlF3 is 1.12.
[0053]The performances of the electrolyte in this embodiment are measured and the measurement result is that the liquidus temperature of the electrolyte in this embodiment is 640° C. The electrolyte has an electric conductivity of about 1.6 Ω−1·cm−1, a density of about 2.03 g / cm3 and an alumina saturation concentration of 5%.
[0054]The process using the electrolyte in the present invention for aluminum electrolysis is as follows:
(1) by means of the anode 3 and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| liquidus temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| electrolysis temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com