Recombinant factor h and variants and conjugates thereof
a technology of recombinant factor and variant, applied in the field of recombinant factor h and variants and conjugates thereof, can solve the problems of logistical and technical difficulties, health risks, and the expected amount of inadequate fh variants, and achieves the effect of not being easy to achiev
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Attempted Expression of Non-Codon-Optimised DNA Encoding FH
[0078]Human FH-encoding DNA was amplified from cDNA, and inserted into the yeast expression vector pPICZalphaB, and KM71 H P. pastoris cells were duly transformed. Cell colonies grew on high antibiotic-containing plates, consistent with the presence of multiple copies of the gene in the transformed cells. We failed, however, to detect (on SDS-PAGE, stained with Coomassie Blue) any evidence of FH expression in mini-scale cultures. Nor was any detectable recombinant FH produced in shaker-flask cultures. We next checked to see if protein expression by transformed cells could be detected under ideal expression conditions (as may be achieved in a one-litre fermentor in which oxygen and nutrient levels are maintained at near-optimal levels) and by using more sensitive detection methods (Western-dot-blot, see FIG. 2); notwithstanding these steps and even with the additional use of a larger-scale (three-litre) fermentation, no recom...
example 2
Development, Purification and Characterisation of Codon-Optimised Human Factor H
[0082]Codon optimisation aimed at human FH expression in P. pastoris was carried out by consultation between the inventors and Geneart (Regensburg, Germany) using their proprietary techniques and GeneOptimizer® software.
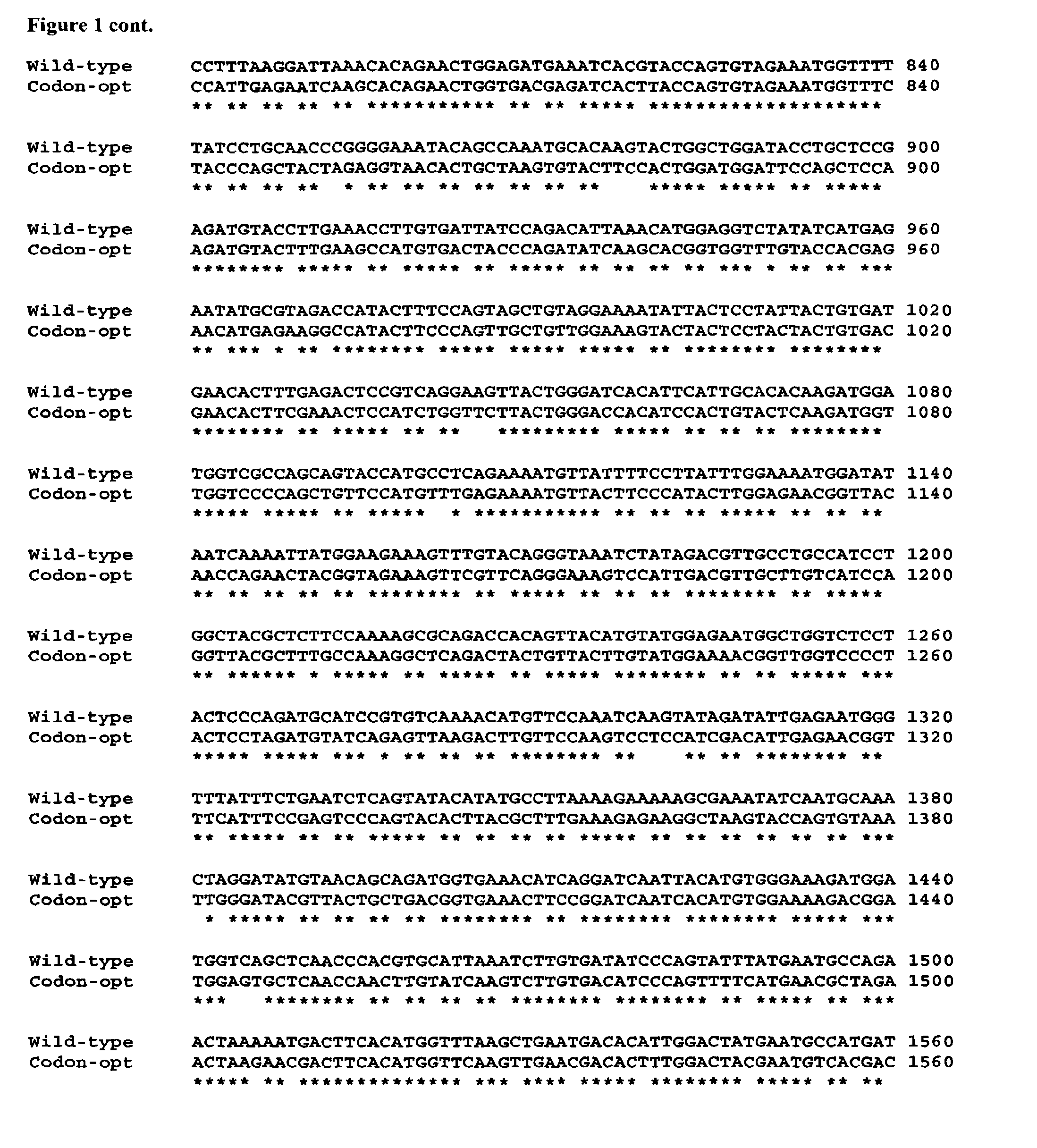
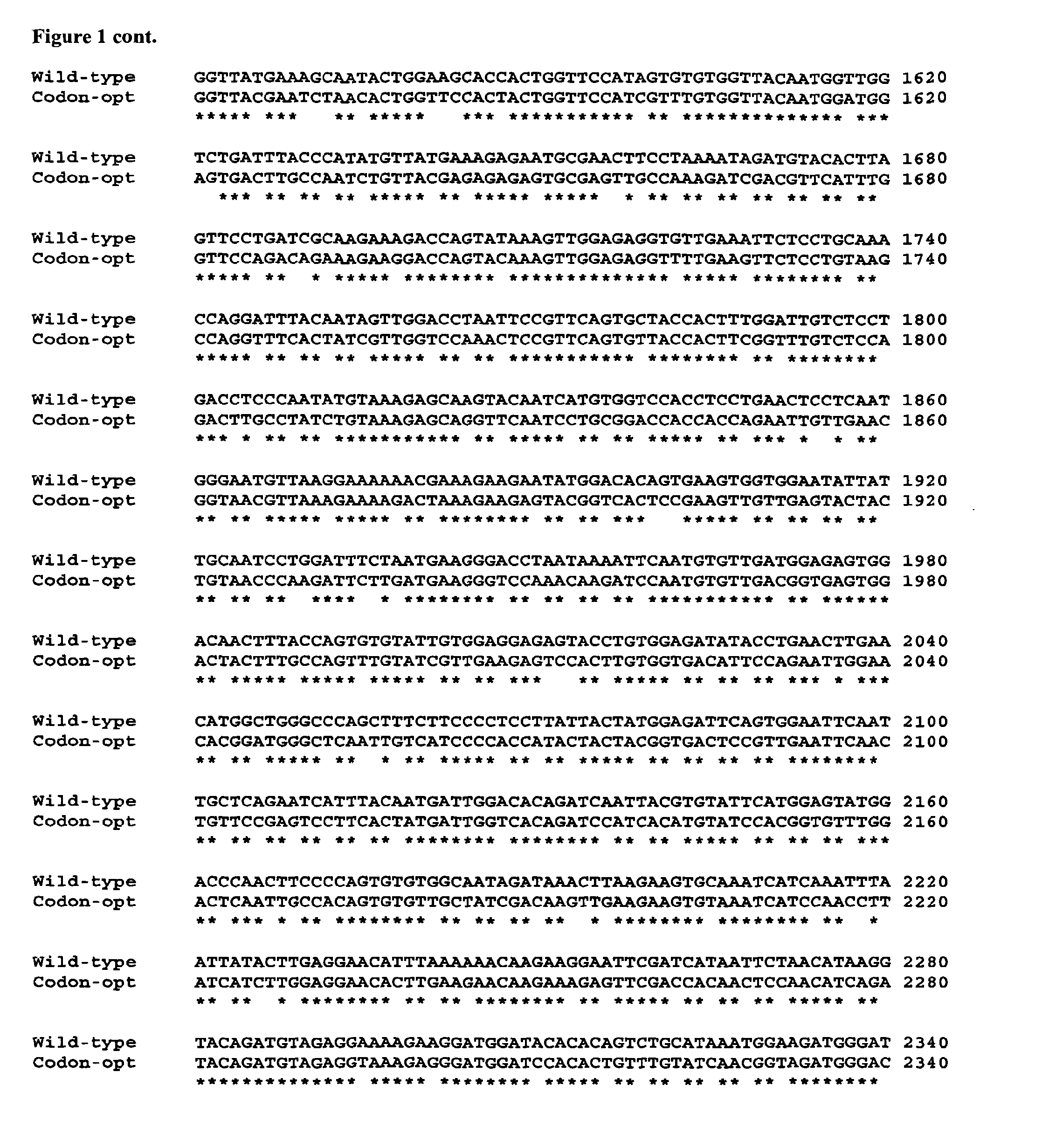
[0083]The nucleic acid sequence of a codon-optimised form of human FH, for expression in P. pastoris, is significantly different (it has 76% sequence identity) to the native DNA sequence (see FIG. 1).
[0084]The codon-optimised DNA sequence was synthesised by Geneart and then cloned into an Invitrogen-purchased P. pastoris-based expression vector, pPICZ alpha B-vector, which had been restricted using appropriate restriction enzymes.
[0085]The vector was transformed into E. coli in order to amplify the DNA, yielding several 10s of μg of plasmid DNA. This was purified, linearised (to enhance homologous recombination) and then transformed (using electroporation) into P. pastoris strain, KM71 H....
example 3
Further Development of Human and Mouse FH Variants Using Codon-Optimised DNA; Elaboration to Enhance Therapeutic Efficacy
[0089]In a first step, a set of 11 plasmid vectors (vector numbers 1 through 11) was designed by the inventors (FIGS. 4A and 4B) in order to further exemplify the utility and versatility of expression of a synthetic codon-optimised gene in P. pastoris. This set of vectors was designed so as to allow “cutting and pasting” of DNA encoding FH between vectors so as to maximise the number of secretion pathways that could be easily explored for each of the targeted FH variants. The aim was to produce mouse FH in addition to human FH, since mouse FH is needed for trials in mice.
[0090]In a second step, the 11 DNA inserts (see FIGS. 5A and 5B for sequence information) intended for codon optimisation were designed by the inventors based on (i) the desired amino acid residue sequences, (ii) the requirement for suitable endonuclease restriction sites, (iii) the incorporation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com