Drug mixing and delivery system and method
a technology of drug mixing and delivery system, applied in the direction of drug composition, peptide/protein ingredient, metabolic disorder, etc., can solve the problems of hives, anaphylactic shock, sharp drop in blood pressure, etc., to improve stability, potency and/or chiral stability, and long shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
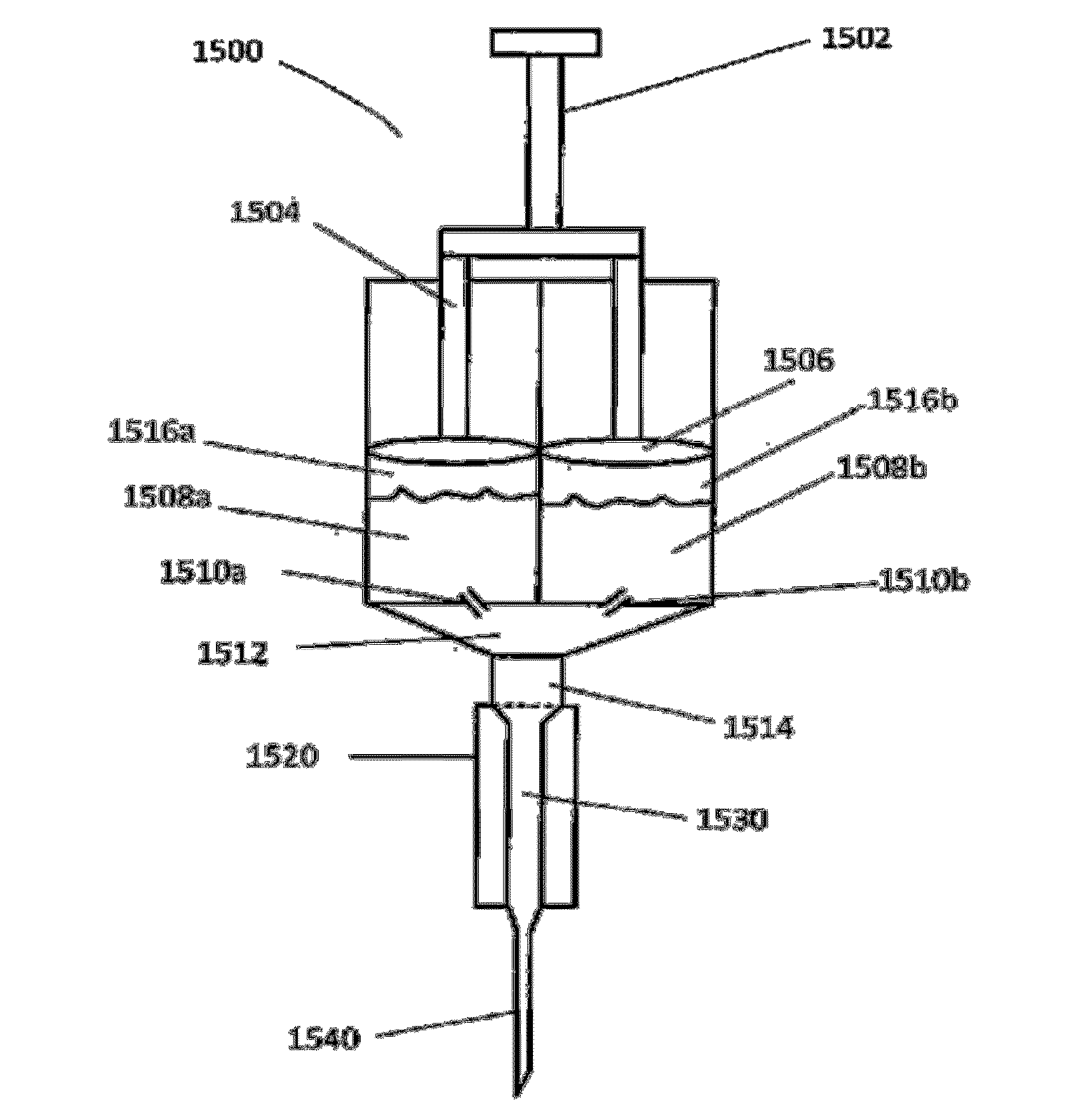
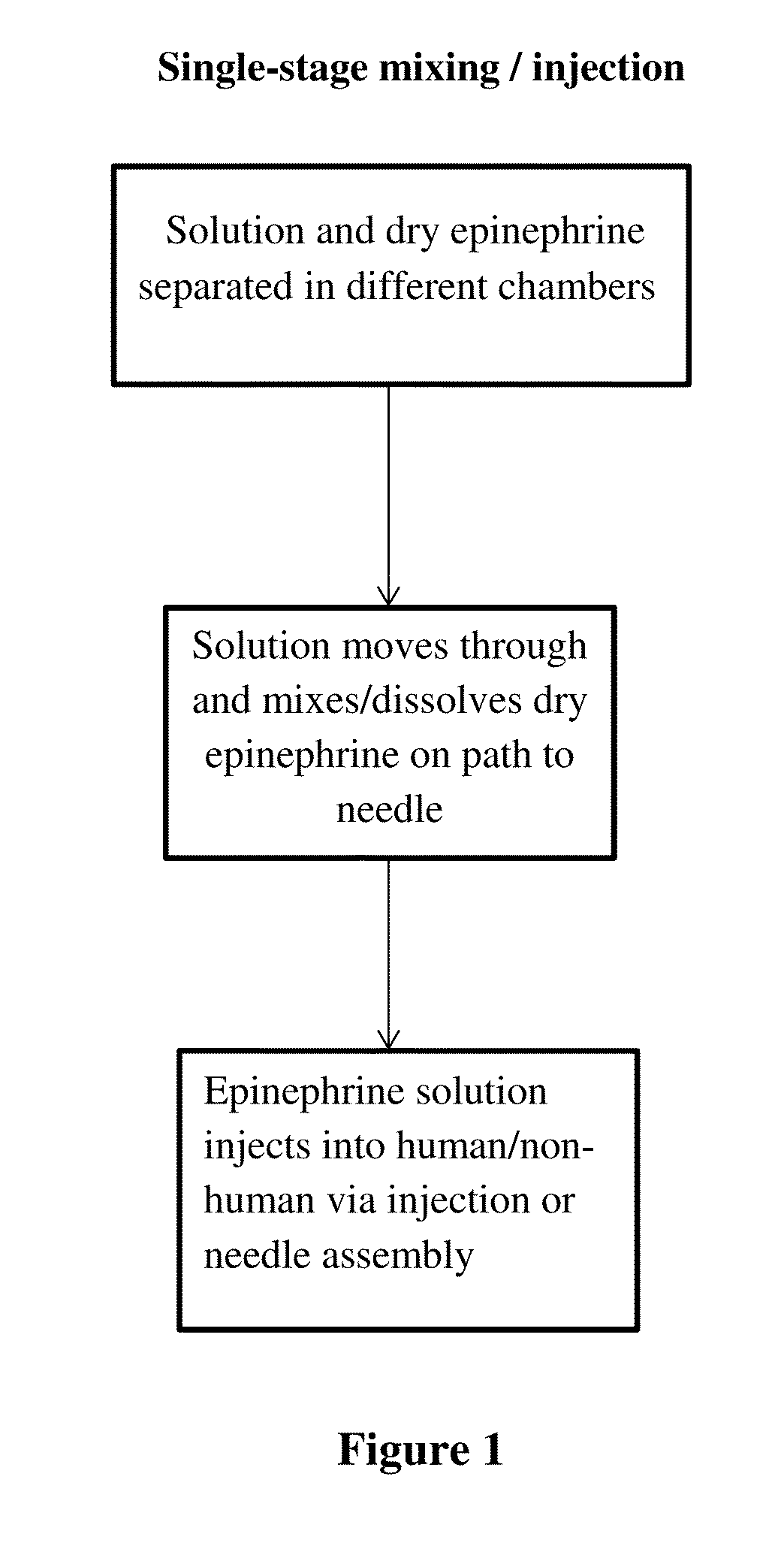
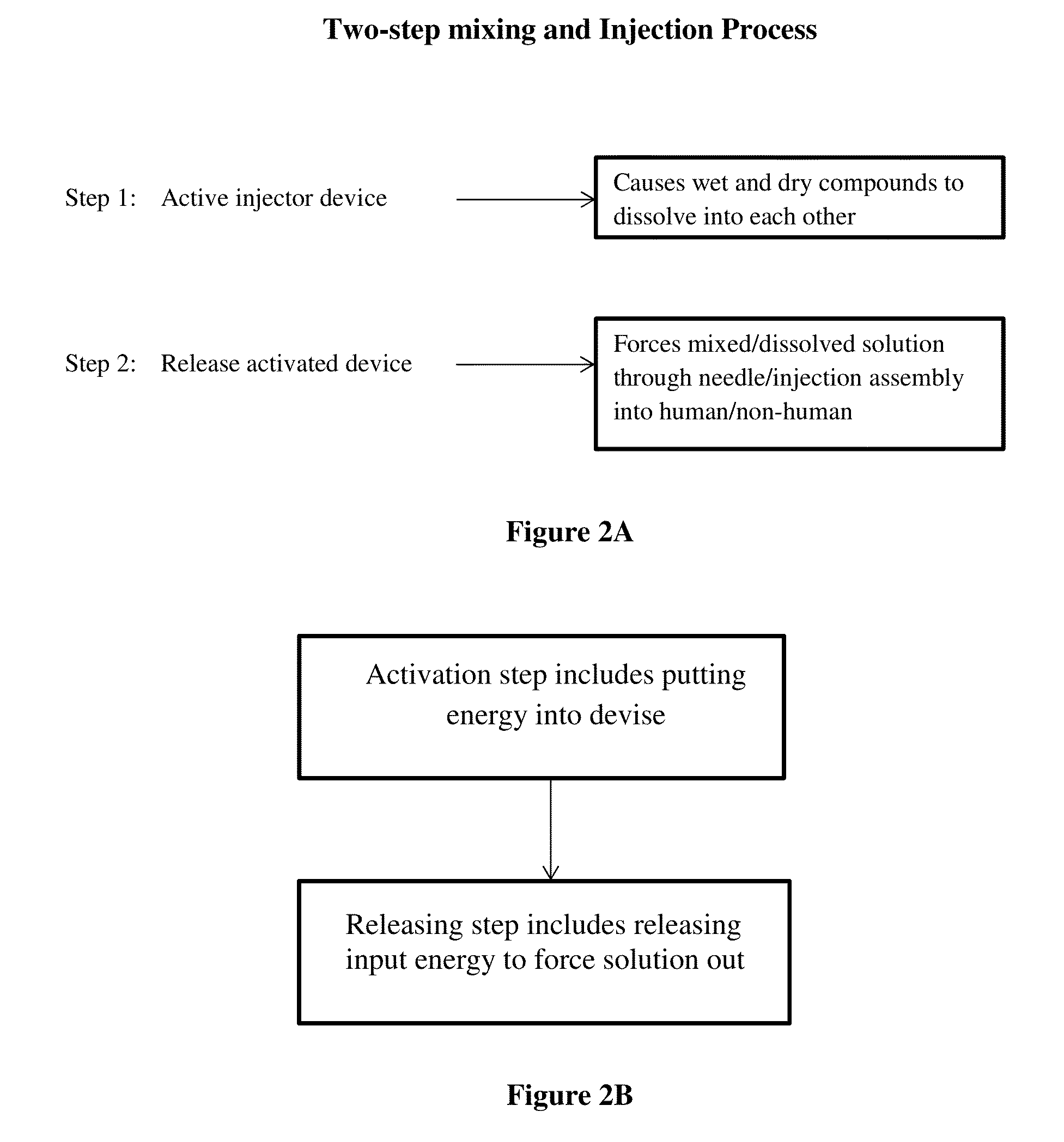
[0035]Provided herein are methods of preparing a medical solution comprising a therapeutic agent. In some embodiments, aspects of the invention relate to stabilizing a drug and making it less susceptible to temperature-induced degradation, by preparing a dry composition (e.g., a dry salt form) of the drug that can be readily reconstituted (e.g., in the context of an autoinjector) for delivery to a patient.
[0036]The dry composition can be prepared from any suitable method as used in the pharmaceutical formulation. For example, a drug may be chemically derived, lyophilized (freeze-dried) and / or spray dried and / or using any other technique to put the drug and / or medicament into a dry form. However, in some embodiments, it is important that the dried drug be easily and rapidly soluble so that the dry composition can be used in an autoinjector that also contains a liquid component that can be mixed with the dry drug to solubilize it upon activation of the autoinjector (e.g., immediately ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com