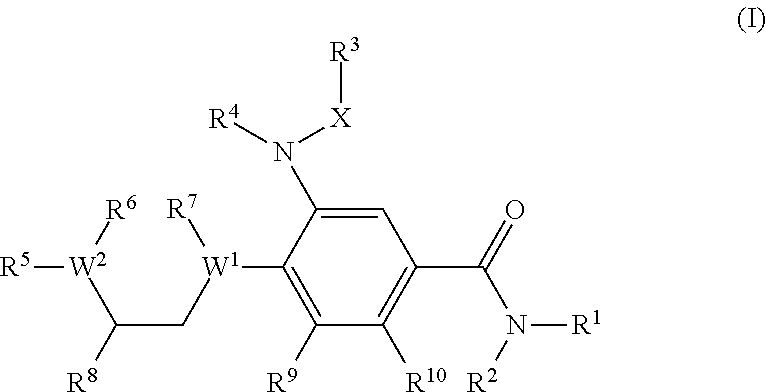
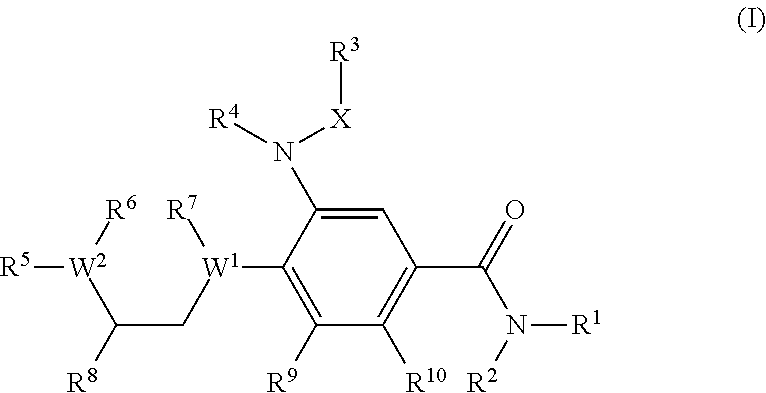
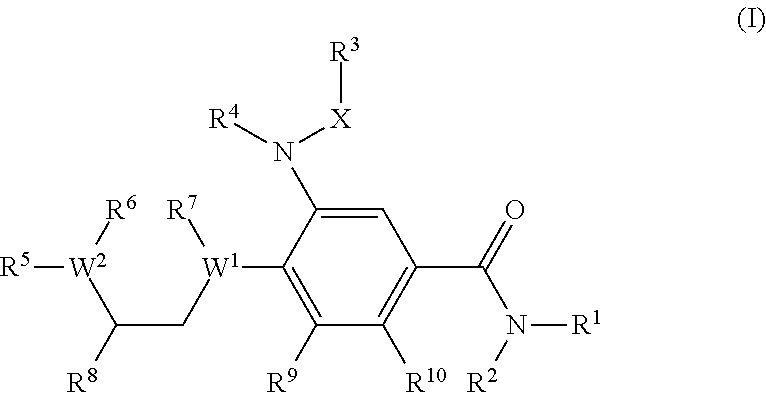
Benzamide derivatives as modulators of the follicle stimulating hormone
a technology of follicle stimulating hormone and derivatives, which is applied in the direction of sexual disorder, drug composition, veterinary instruments, etc., can solve the problems of high cost, limited use of fsh, and high cost of oral dosing, and achieve the effect of high cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthetic Route Towards 2-cyclopropyl-oxazole-4-carboxylic acid [4-chloro-5-[3-(2-oxo-pyrrolidin-1-yl)propylcarbamoyl]-2-(4-o-tolyl-piperazin-1-yl)-phenyl]-amide (1i)
[0219]
[0220]Step 1: 2-Chloro-4-fluoro-benzoic acid 1a (5.0 g, 28.64 mmol) was taken in sulphuric acid (20.0 mL) and cooled to 0° C. Nitric acid (10.0 ml) was added to this very carefully and slowly. The reaction was stirred at 0-25° C. for 6 h. The white solid was filtered and washed with water and dried at 40° C. under vacuum and used in the next step.
[0221]Step 2: To a solution of 2-chloro-4-fluoro-5-nitro-benzoic acid 1b (2.0 g, 9.1 mmol) in DMF (20.0 mL) was added potassium carbonate (2.51 g, 18.21 mmol) slowly followed by 1-o-tolyl-piperazine 1e (1.9 g, 10.93 mmol) and the reaction was stirred at room temperature for 16 h. Water was added carefully and the solution was acidified to pH 5.0 using 1N HCl to give yellow solid which was filtered and dried.
[0222]Step 3: To a solution of 2-chloro-5-nitro-4-(4-o-tolyl-pipe...
example 2a
Synthetic Route Towards 2-cyclopropyl-oxazole-4-carboxylic acid [4-methoxy-5-[3-(2-oxo-pyrrolidin-1-yl)-propylcarbamoyl]-2-(4-o-tolyl-piperazin-1-yl)-phenyl]-amide 2c
[0227]
[0228]Step 1: To a solution of 2-chloro-5-nitro-N-[3-(2-oxo-pyrrolidin-1-yl)-propyl]-4-(4-o-tolyl-piperazin-1-yl)-benzamide 1f (350.0 mg, 0.70 mmol) in methanol (10.0 ml) was added sodium methoxide (378.1 mg, 7.0 mmol) and the reaction was stirred at 45° C. for 6 h. The reactions mixture was concentrated, water was added carefully and extracted with ethyl acetate. Organic layer was washed with water and brine and concentrated. Crude 2a was purified on silica gel using dichloromethane / MeOH (10%) to give product (248 mg, 71%).
[0229]Step 2: 2-Methoxy-5-nitro-N-[3-(2-oxo-pyrrolidin-1-yl)-propyl]-4-(4-o-tolyl-piperazin-1-yl)-benzamide 2a (350.0 mg, 0.706 mmol) was taken in ethanol and evacuated and nitrogen purged. This was added to a flask containing Pd / C (5 wt %) (300.6 mg, 0.141 mmol) under nitrogen. Flask was evacu...
example 2b
Synthetic Route Towards 2-cyclopropyl-oxazole-4-carboxylic acid [4-hydroxy-5-[3-(2-oxo-pyrrolidin-1-yl)-propylcarbamoyl]-2-(4-o-tolyl-piperazin-1-yl)-phenyl]-amide 2d
[0233]
[0234]To a solution of 2-cyclopropyl-oxazole-4-carboxylic acid [4-methoxy-5-[3-(2-oxo-pyrrolidin-1-yl)-propylcarbamoyl]-2-(4-o-tolyl-piperazin-1-yl)-phenyl]-amide 2c (40.0 mg, 0.067 mmol) in pyridine (5.0 mL) was added magnesium bromide (49.0 mg, 0.268 mmol). The reaction was stirred at 130° C. for 16 h. Cooled, concentrated and the crude 2d was dissolved in methanol / water mixture and purified on preparative HPLC using methanol / water as eluent.
[0235]LCMS (ESI) 587 (M+H);
[0236]1H NMR (400 MHz, DICHLOROMETHANE-d2) δ ppm 1.14 (d, J=2.34 Hz, 4H) 1.70-1.85 (m, 2H) 2.00-2.14 (m, 3H) 2.34 (s, 3H) 2.42 (d, J=8.30 Hz, 2H) 3.04-3.19 (m, 8H) 3.31-3.45 (m, 4H) 6.76 (s, 1H) 6.92-7.04 (m, 1H) 7.18 (d, J=13.42 Hz, 3H) 7.85-7.99 (m, 1H) 8.10 (s, 1H) 8.70 (s, 1H) 9.41-9.60 (m, 1H) 12.83 (s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com