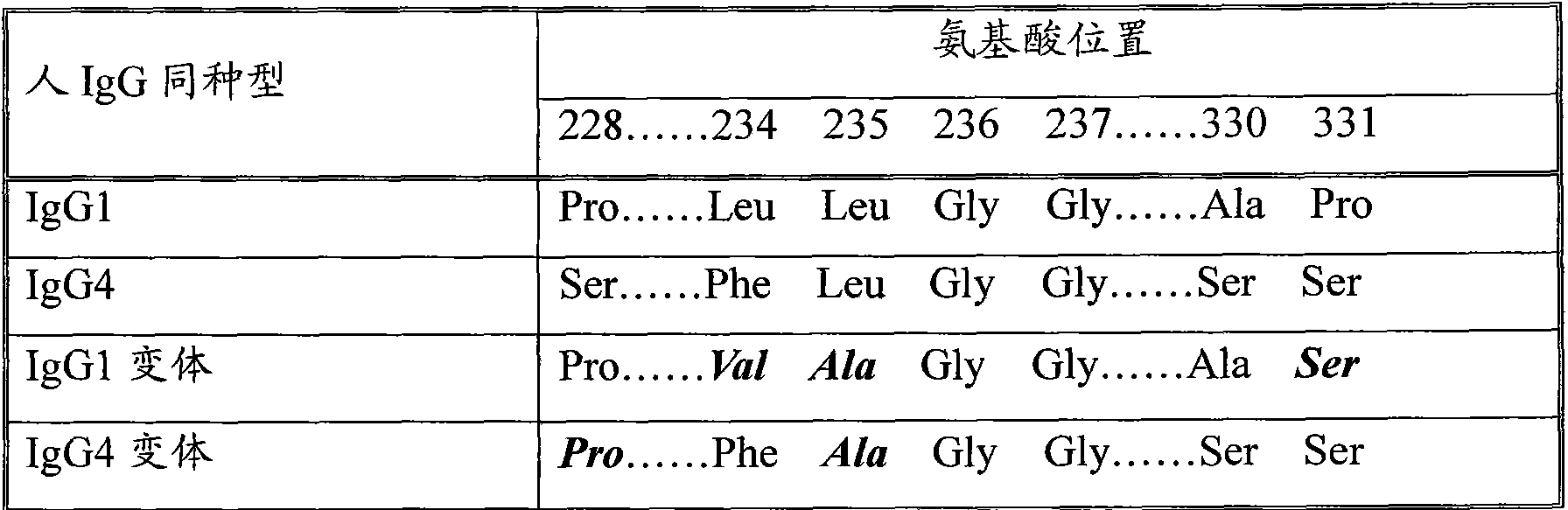
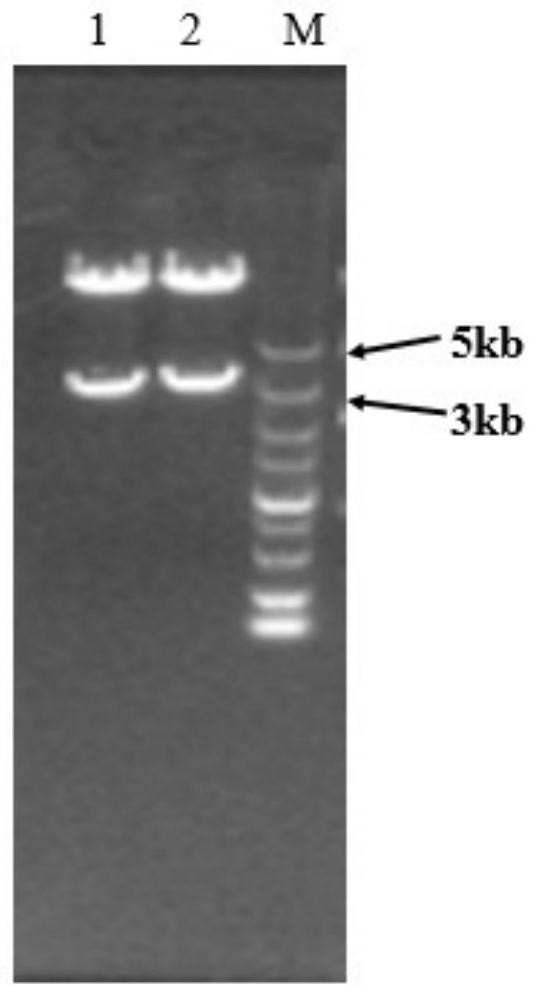
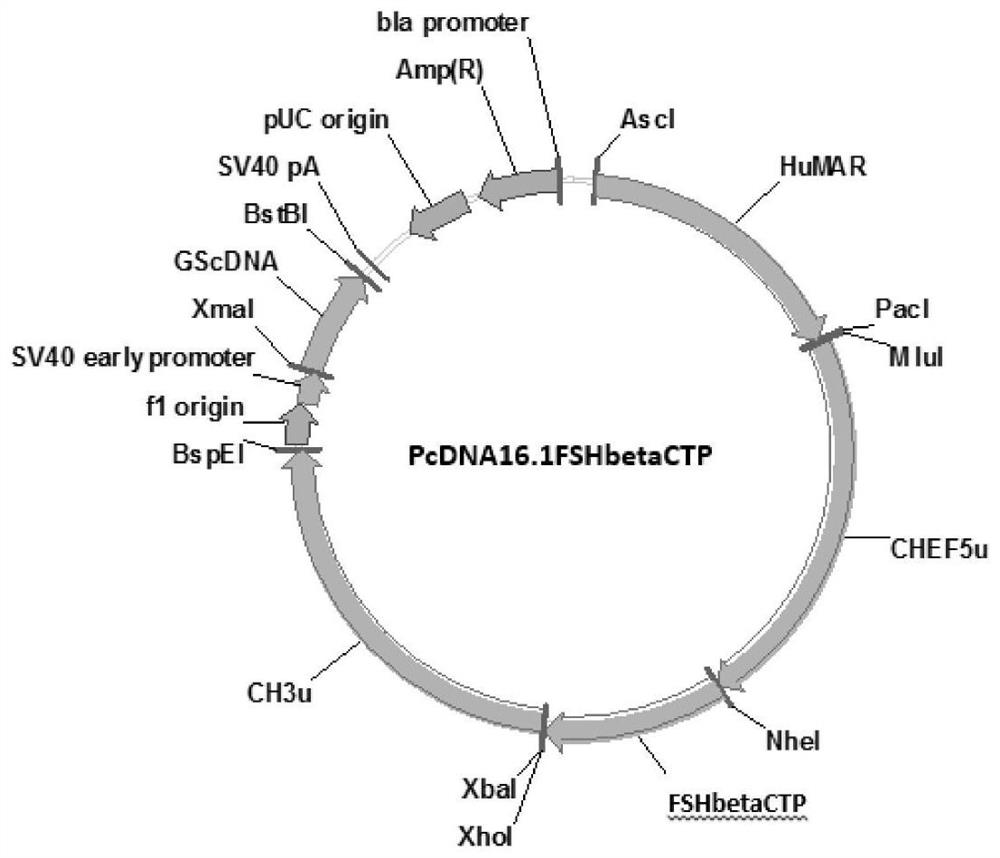
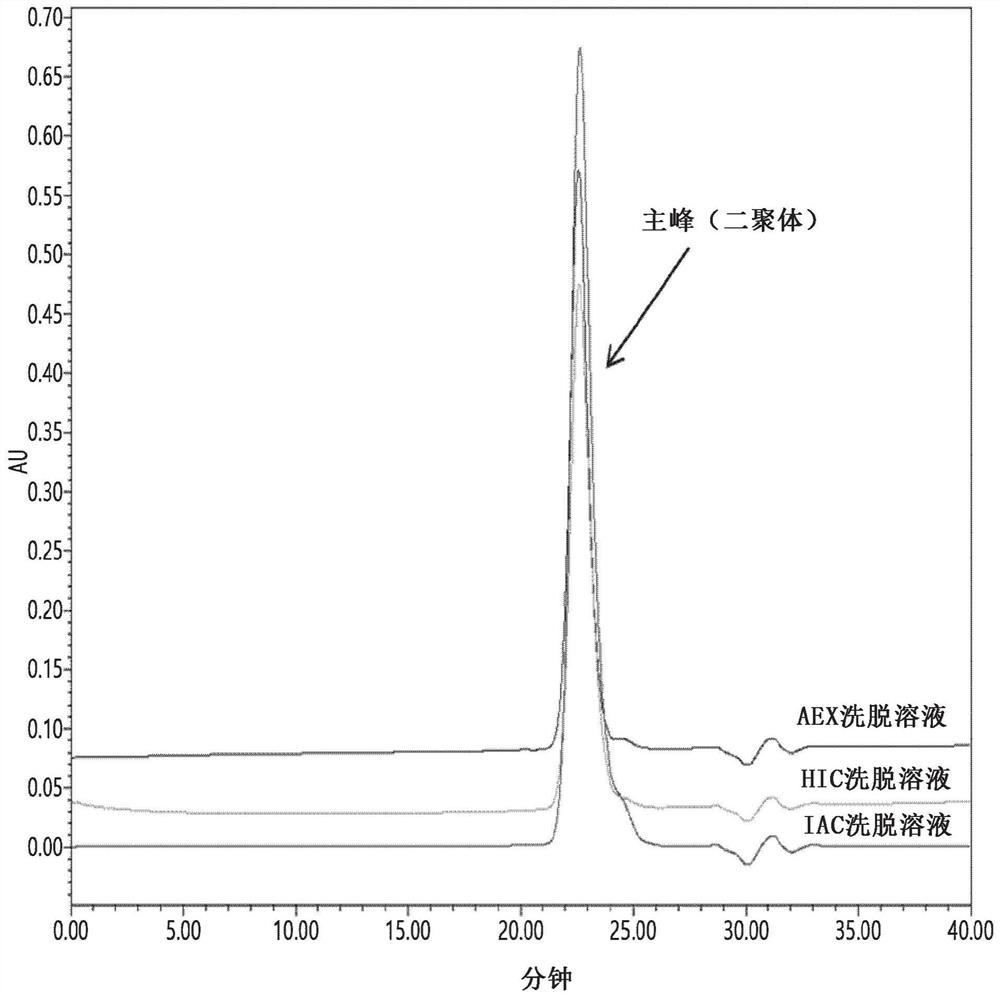
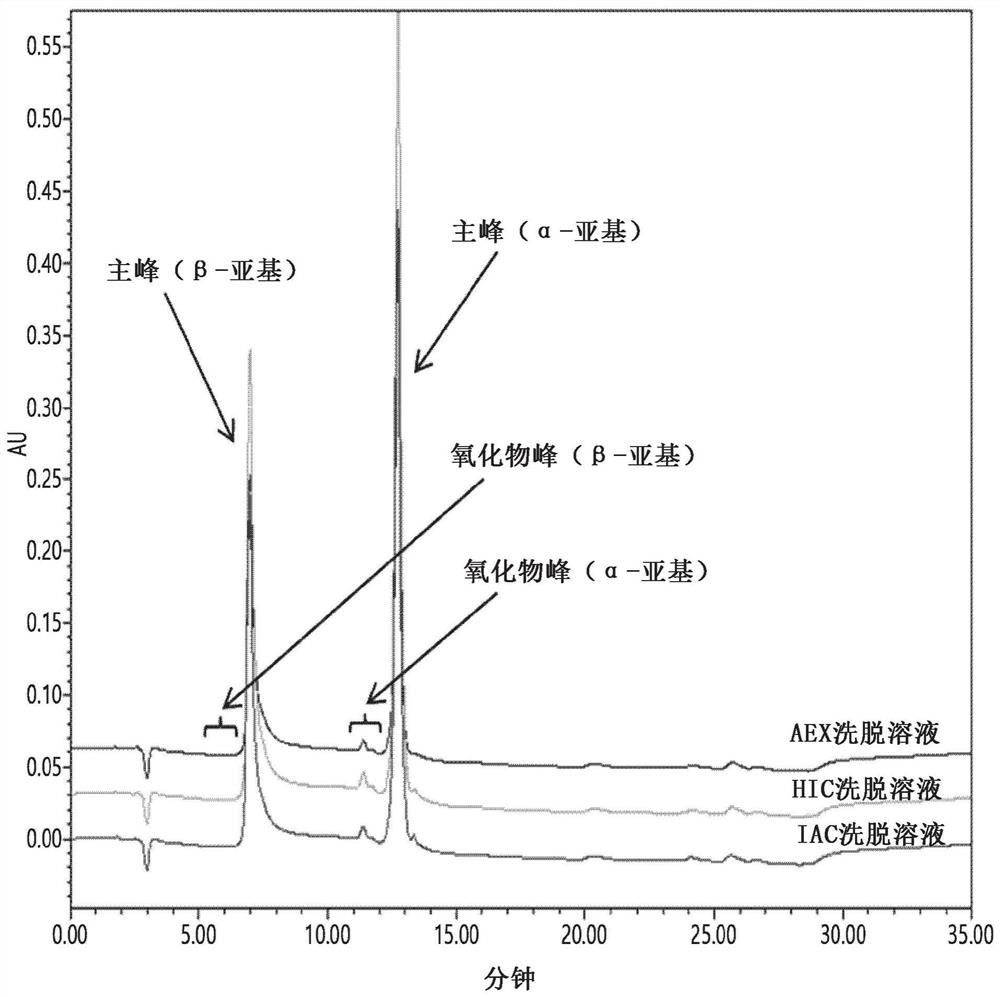
Patents
Literature
49 results about "FSH - Follicle-stimulating hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polymer-based compositions for sustained release
InactiveUS20040028733A1Reduced activityReduce aggregationPowder deliveryPeptide/protein ingredientsFollicle-stimulating hormoneSpermatogenesis
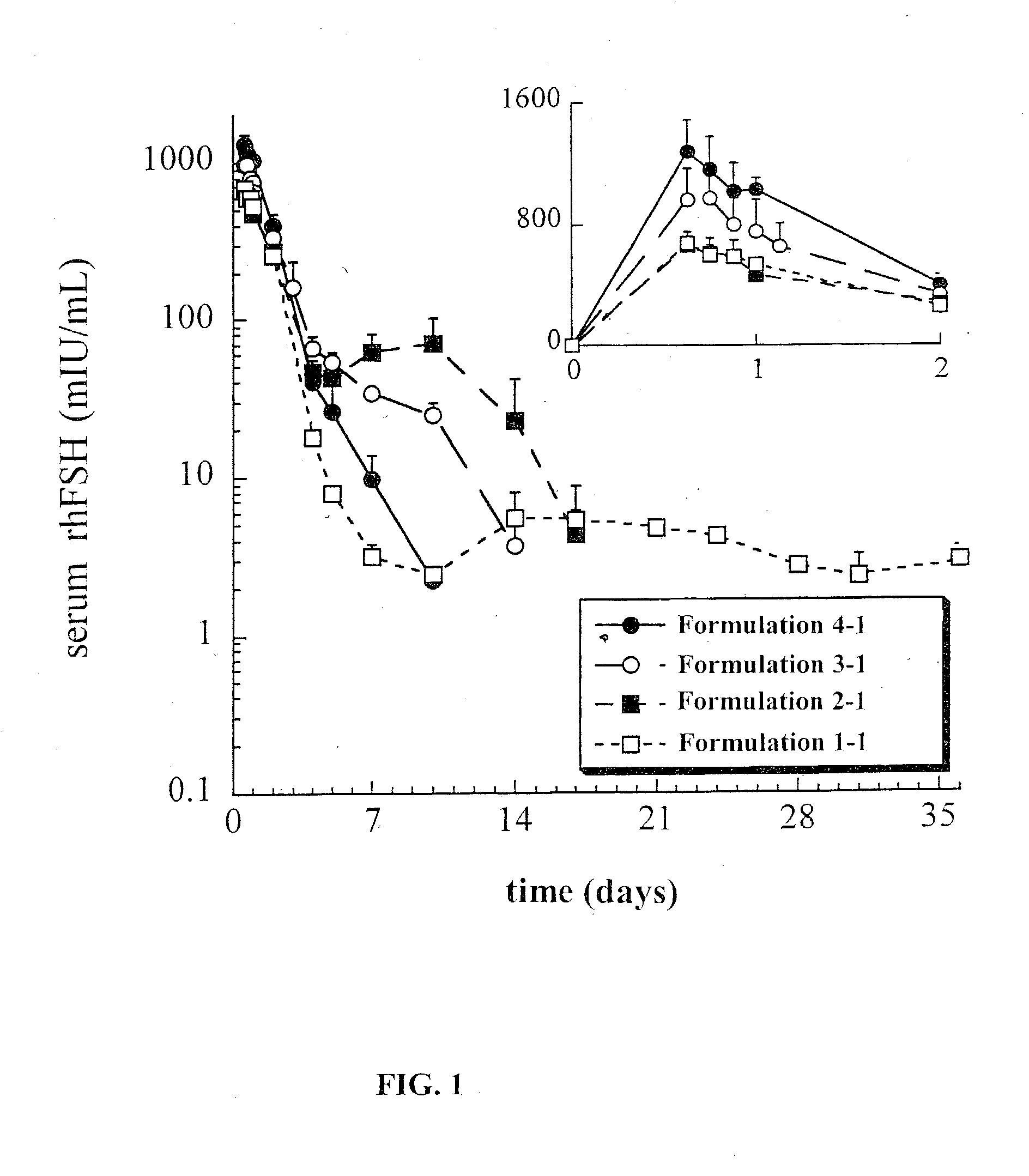
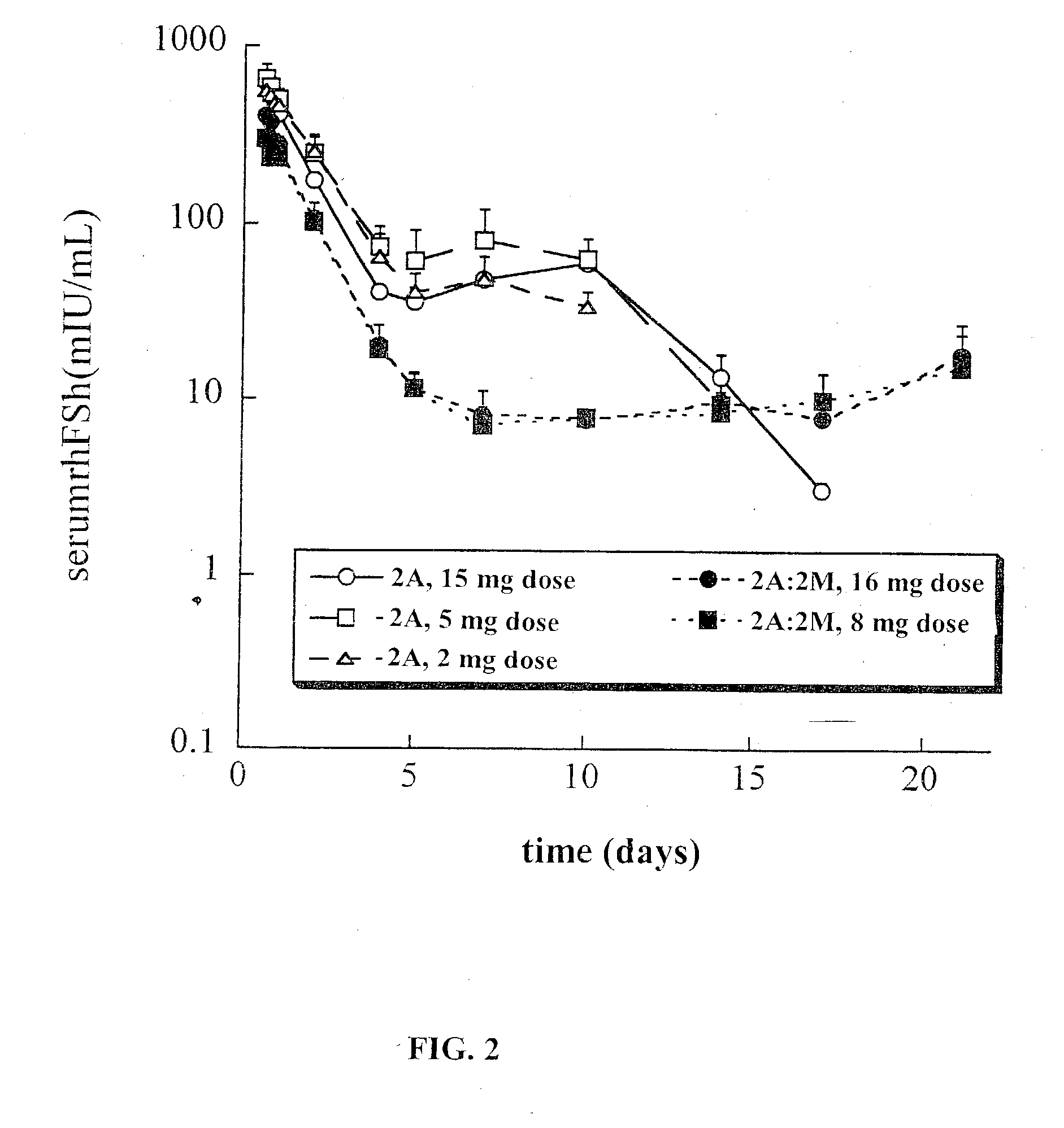
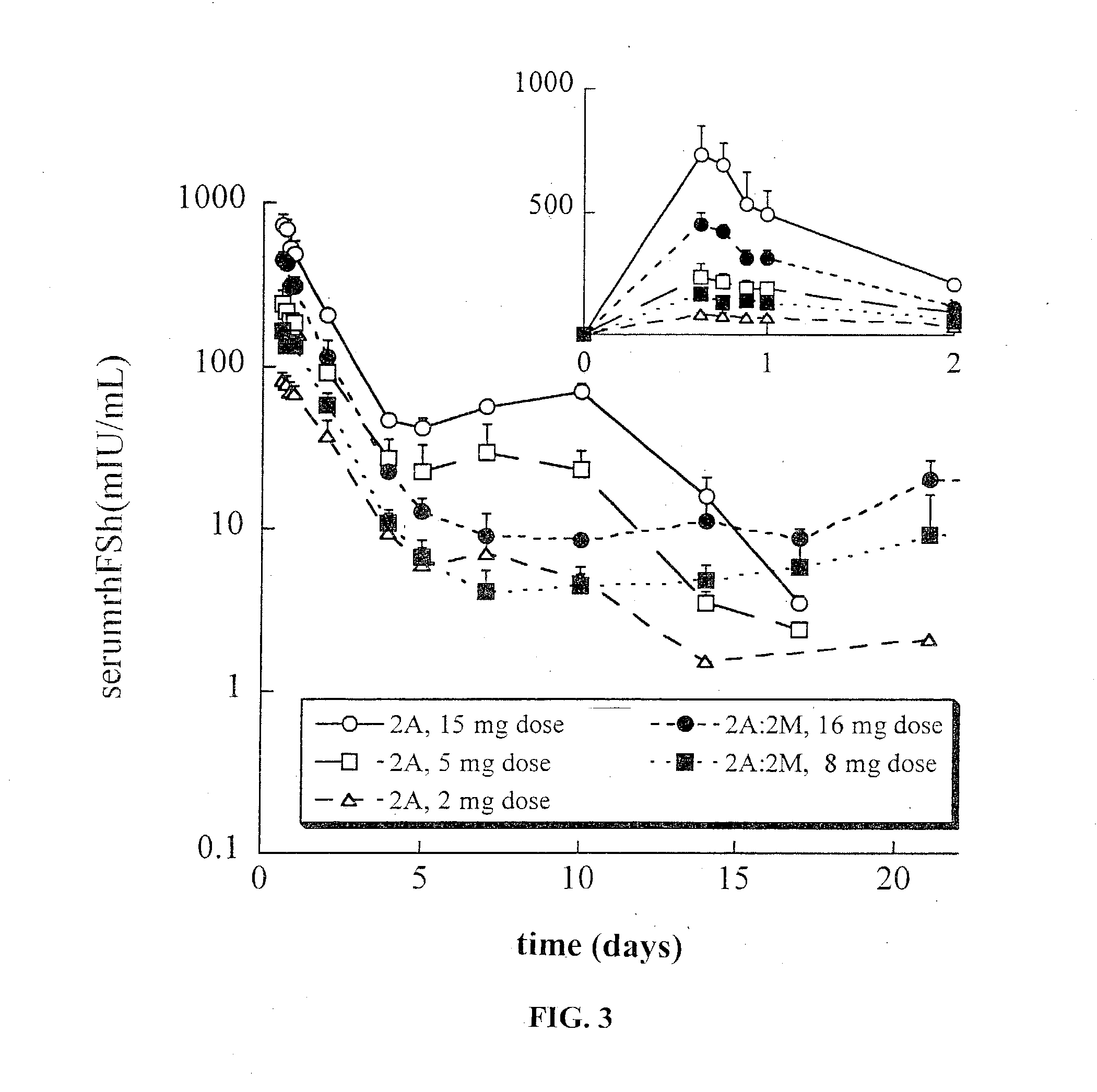
This invention relates to sustained release compositions, and methods of forming and using said compositions, in particular for the sustained release of Follicle Stimulating Hormone (FSH). The sustained release compositions comprise a polymeric matrix of a biodegradable biocompatible polymer and stabilized FSH. The method of the invention for forming a sustained release composition includes, dissolving a biodegradable biocompatible polymer in a polymer solvent to form a polymer solution; adding biologically active stabilized FSH; removing the solvent; and solidifying the polymer to form a polymer matrix containing stabilized FSH dispersed therein. Also described is a method for providing a therapeutically effective amount of stabilized FSH in a patient in need of for a sustained period comprising administering to the patient a dose of the sustained release compositions of the invention. The sustained release composition of FSH can be used to promote maturation of follicles, promote spermatogenesis and to treat fertility disorders.
Owner:MERCK SERONO INT
Follicle stimulating hormone-glycosylation analogs
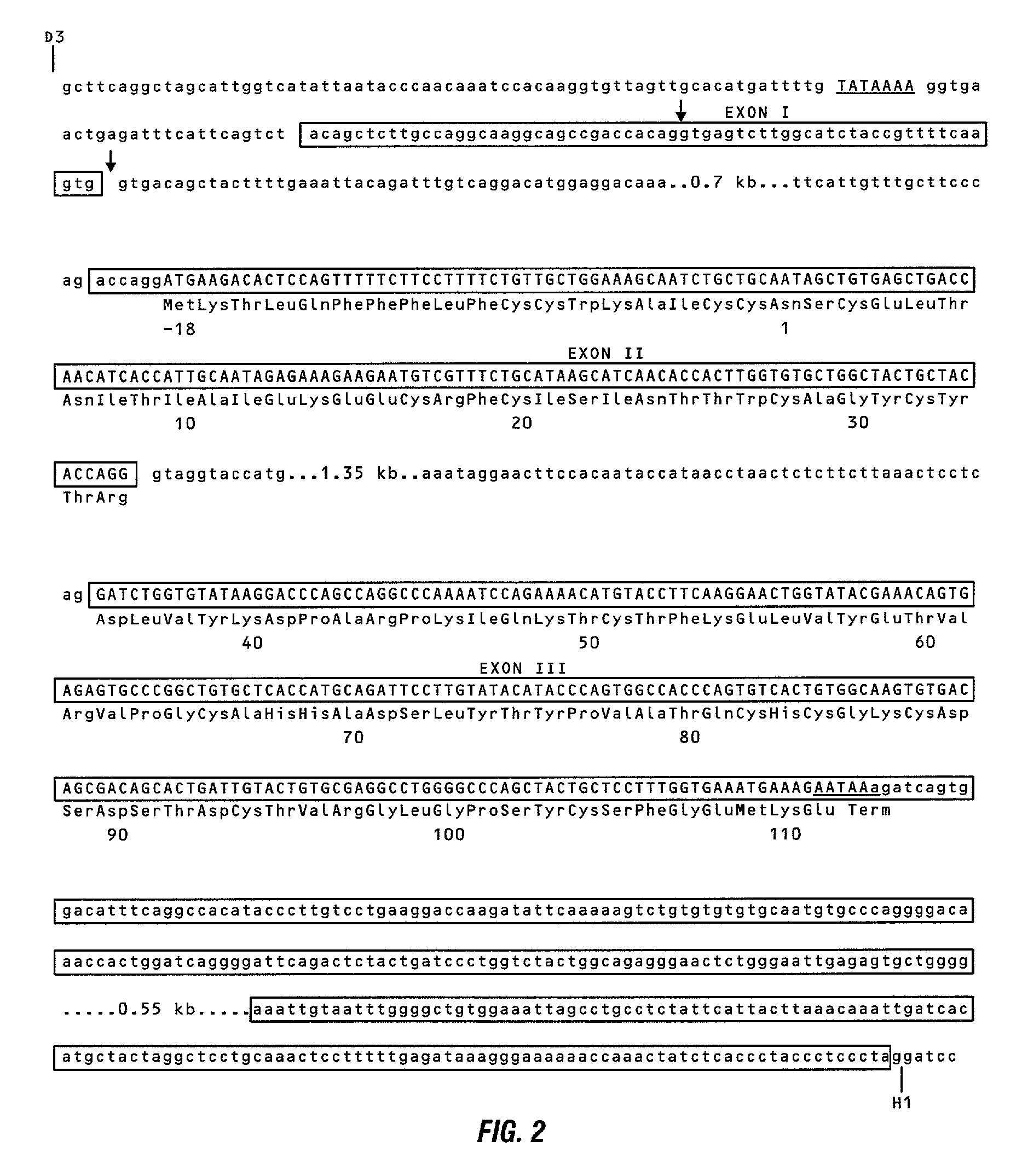
InactiveUS20010007757A1Sugar derivativesPeptide/protein ingredientsDimerHuman follicle stimulating hormone
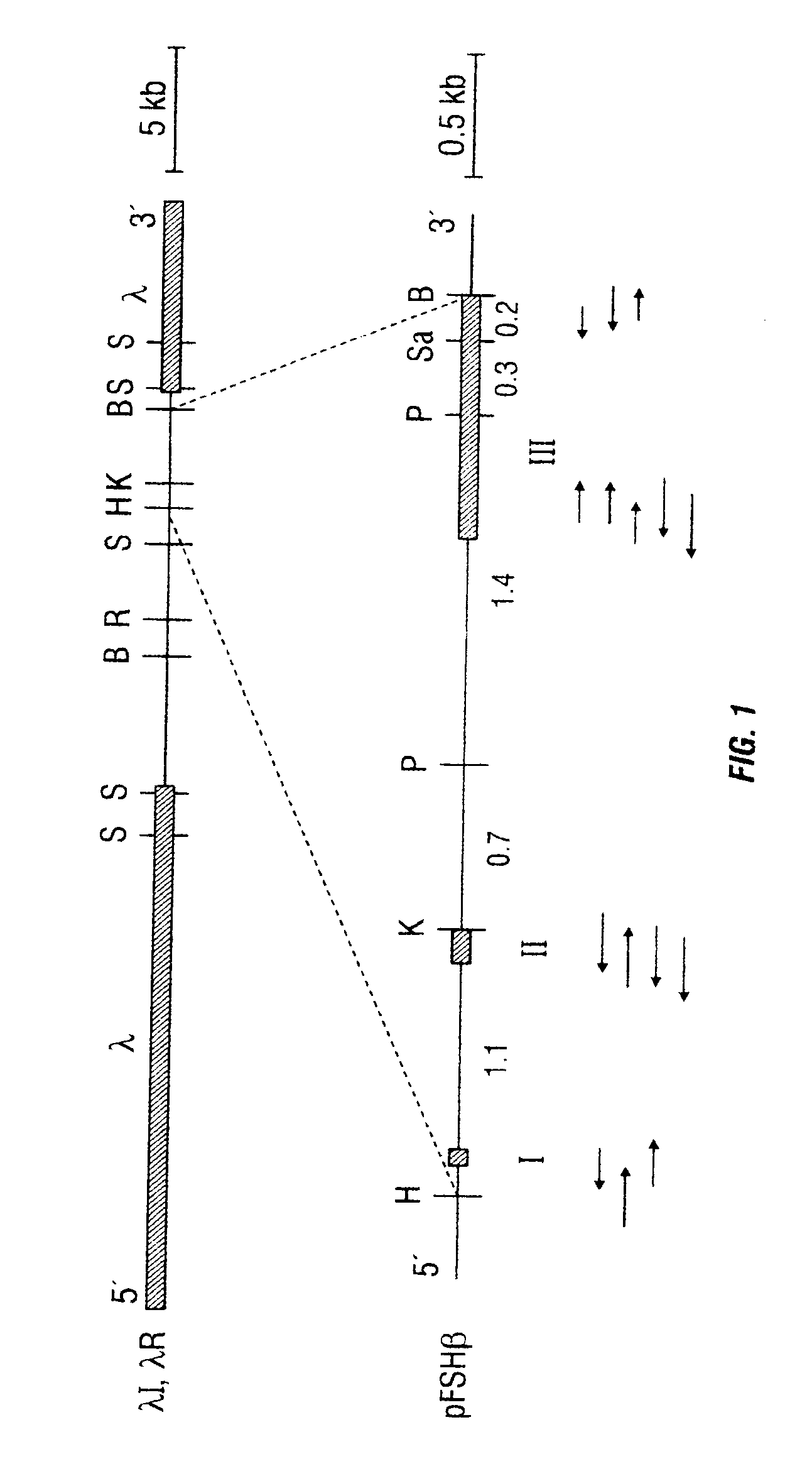
The invention provides recombinant native and mutein forms of human follicle stimulating hormone beta subunit (FSH beta) with characteristic glycosylation patterns which are influential in the metabolic activity of the protein. The invention also provides recombinant mutant forms of the human alpha subunit common to FSH, LH, CG, and TSH, to obtain hormones which also have unique glycosylation patterns. Also provided are recombinant materials to produce these subunits separately or together to obtain complete heterodimeric hormones of regulated glycosylation pattern.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Preservative-Free Follicle Stimulating Hormone Solution Delivery Device
ActiveUS20120265136A1Peptide/protein ingredientsAutomatic syringesFollicle-stimulating hormonePreservative free
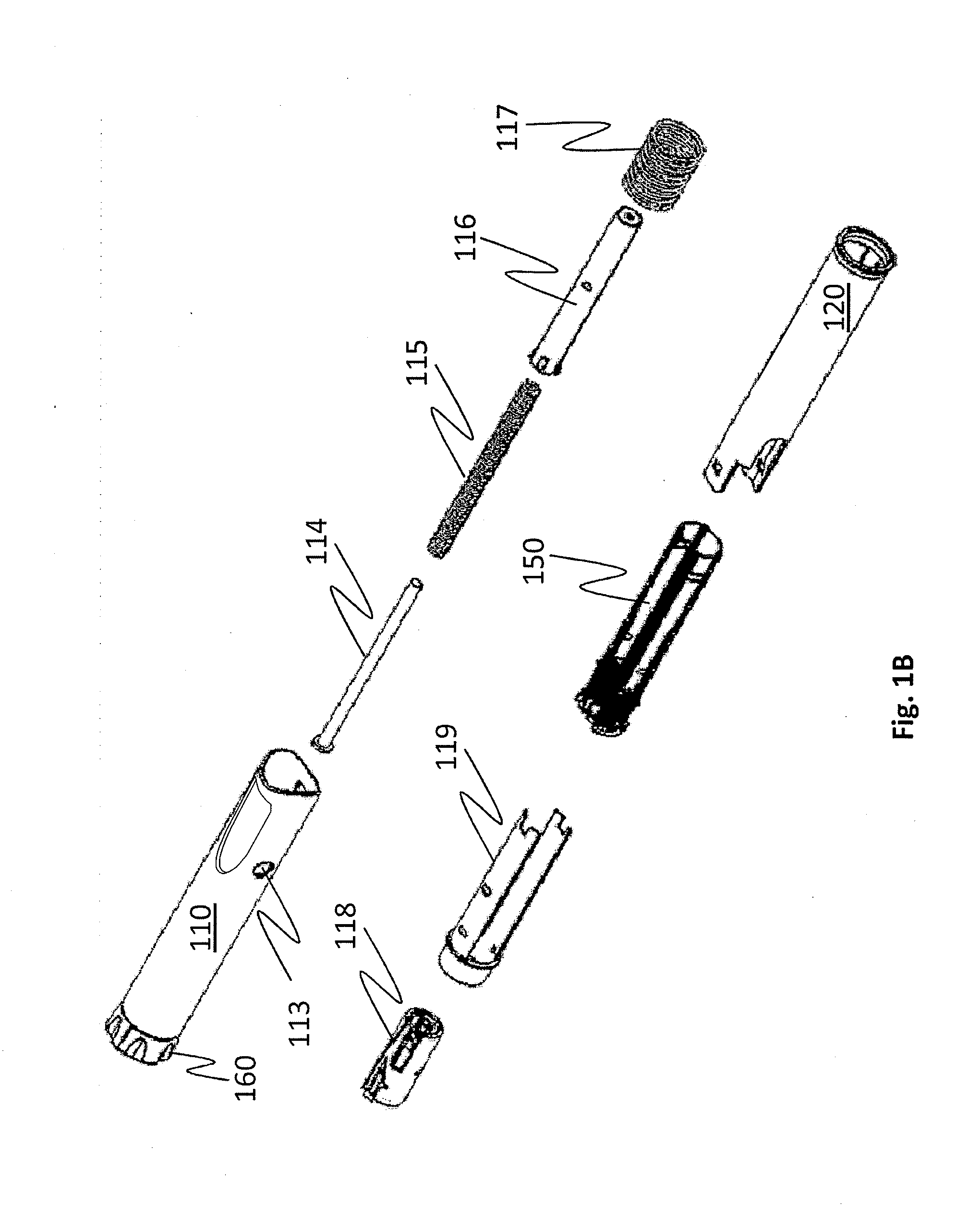
A one-time use device to deliver preservative-free follicle stimulating hormone (FSH) solution is disclosed. The device includes a needle covered by a sliding needle shield, which covers the needle in all modes of the device. The device can be placed into a ready-to-use position in four or fewer user steps. The device has a knob for setting a desired dose of FSH. The knob includes longitudinally spaced elements respectively corresponding to the lock position and the seven or fewer discrete dosing positions. The device locks after one use and cannot be reused thereafter.
Owner:SHL MEDICAL AG
Culture method for high-efficiency human follicle stimulating hormone expression CHO cells
ActiveCN105462909AStable batch-to-batch qualityThe cultivation process is stable and controllableMicroorganism based processesVertebrate cellsBottleHigh survival rate
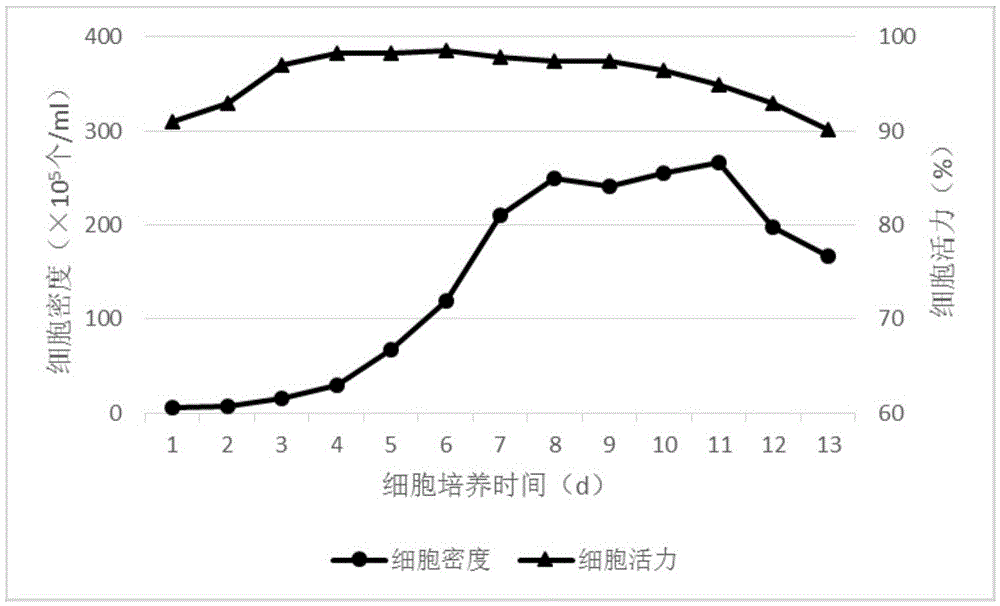
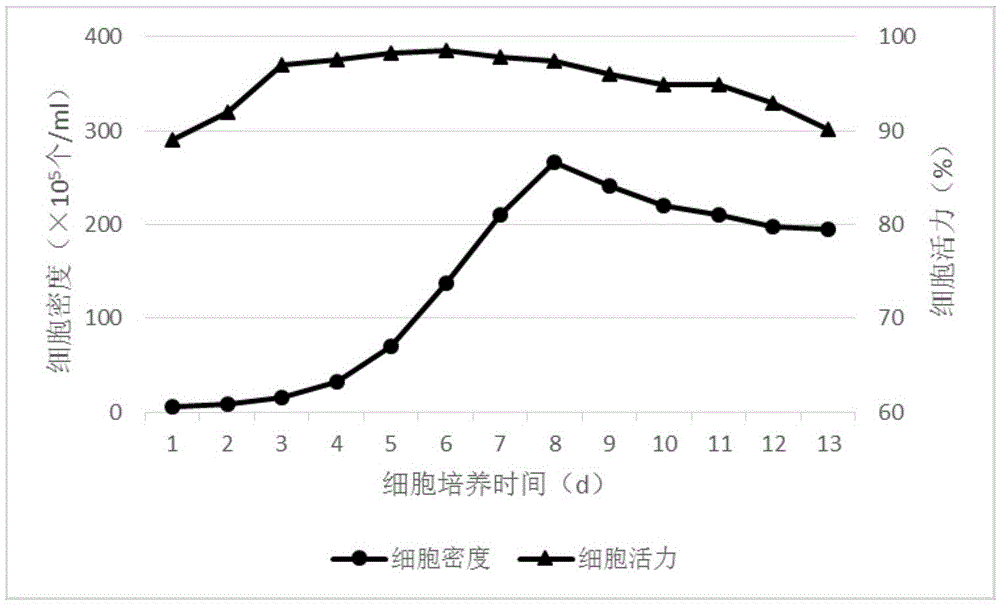
The invention discloses a culture method for high-efficiency human follicle stimulating hormone expression CHO cells and belongs to the technical field of bio-pharmaceuticals. According to the culture method, seed cells are prepared into cell suspension, and subculture is performed after resuspension through a serum-free basal culture medium to obtain seed suspension; the obtained seed suspension is inoculated into a bioreactor to be cultured, the serum-free basal culture medium is fed at regular intervals in the culture process, growth situations of the cells are monitored, glucose solution is supplemented, and the cells are harvested after culture. According to the method, the serum-free basal culture medium is used throughout the process, culture in a fermentation tank is performed after reviving and amplification through bottle shaking, the process flow is simple, the cell culture density is high and can be as high as 4*107 / mL , the cell survival rate reaches more than 98%, maintaining time under high density and a high survival rate is long, the yield of end products can reach 40mg / L, and the method is applicable to industrial production.
Owner:哈药集团股份有限公司 +1
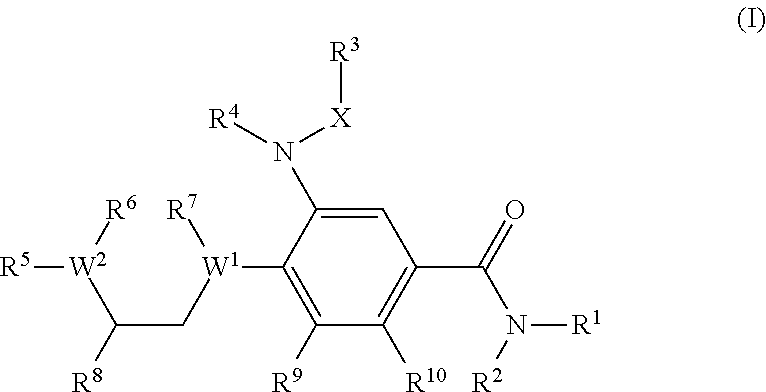
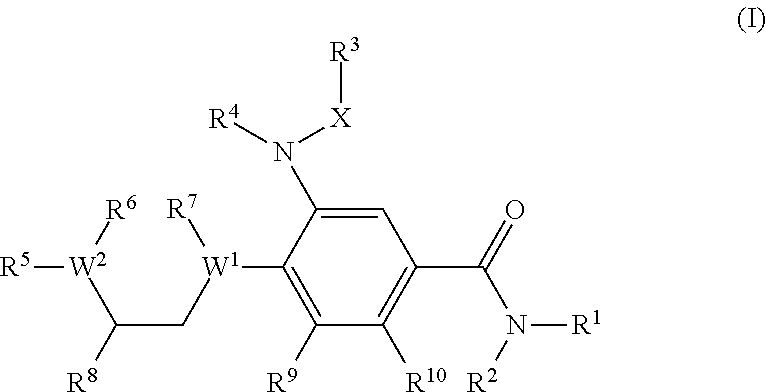
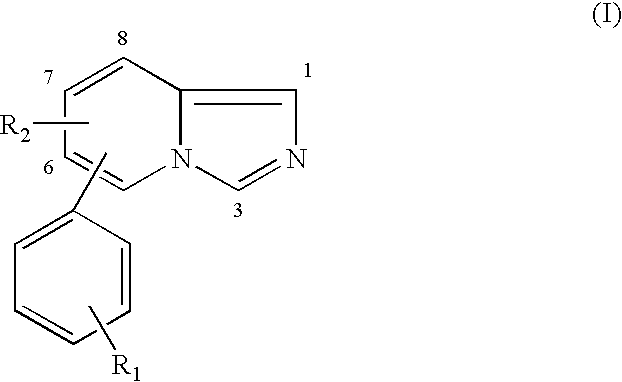
Novel benzamide derivatives as modulators of the follicle stimulating hormone
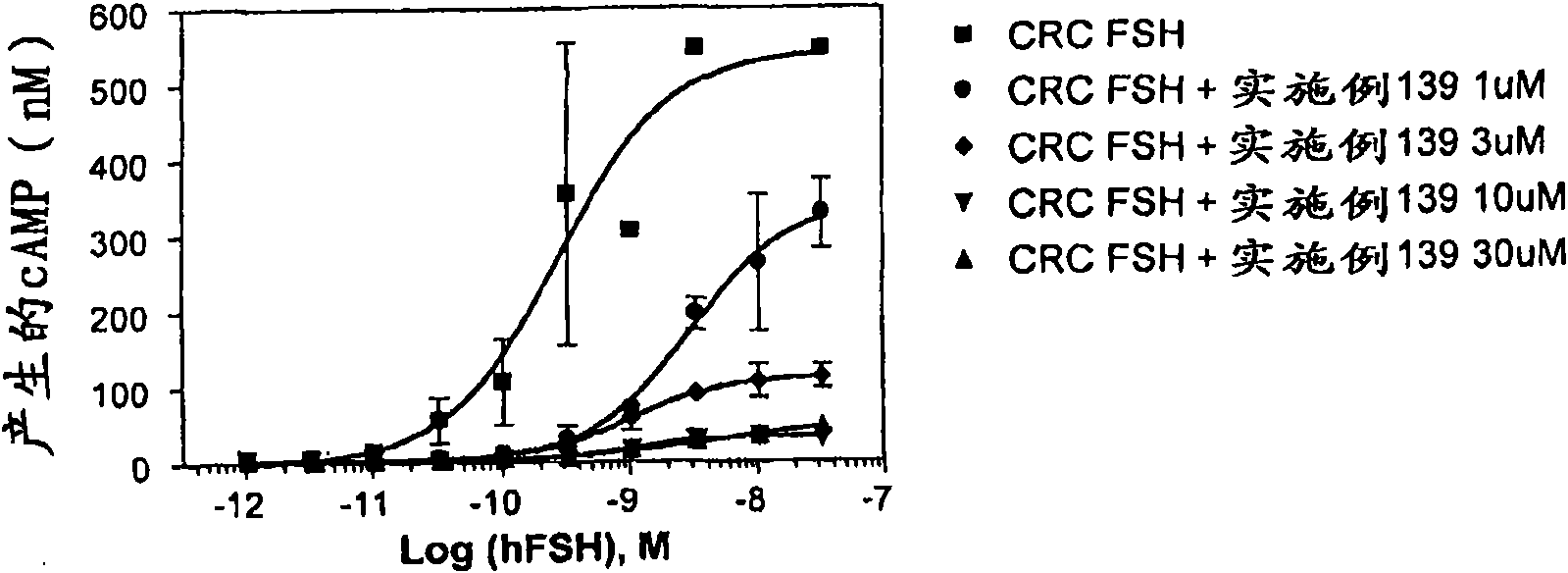
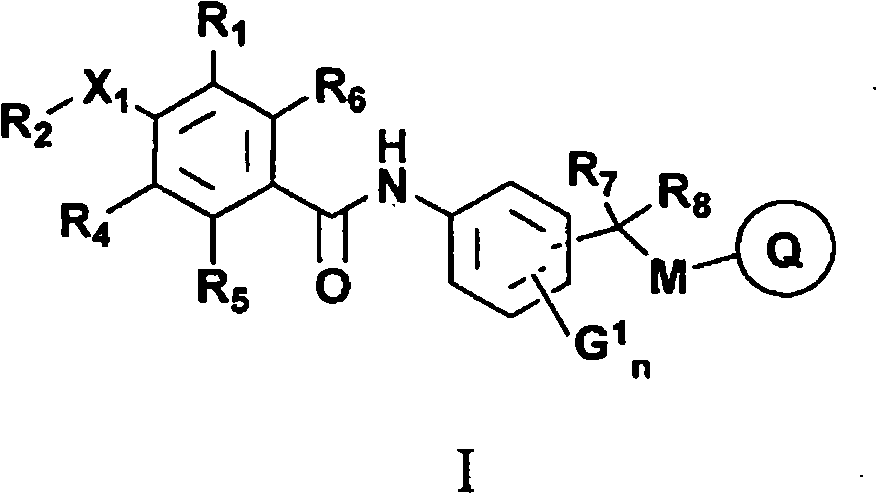
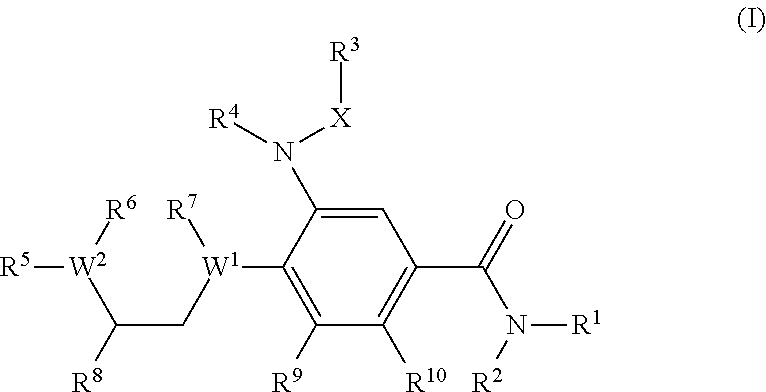
The present invention provides new compounds of formula I, wherein Q, R1, R2, R4, R5, R6, Xi, R7, R8, M and G1 n are defined as in formula I; inventi on compounds are modulators of follicle-stimulating hormone - ('FSH') which are useful for male and female contraception as well as other disorders modu lated by FSH receptor.
Owner:ADDEX PHARM SA
Development of follicle stimulating hormone agonists and antagonists in fish
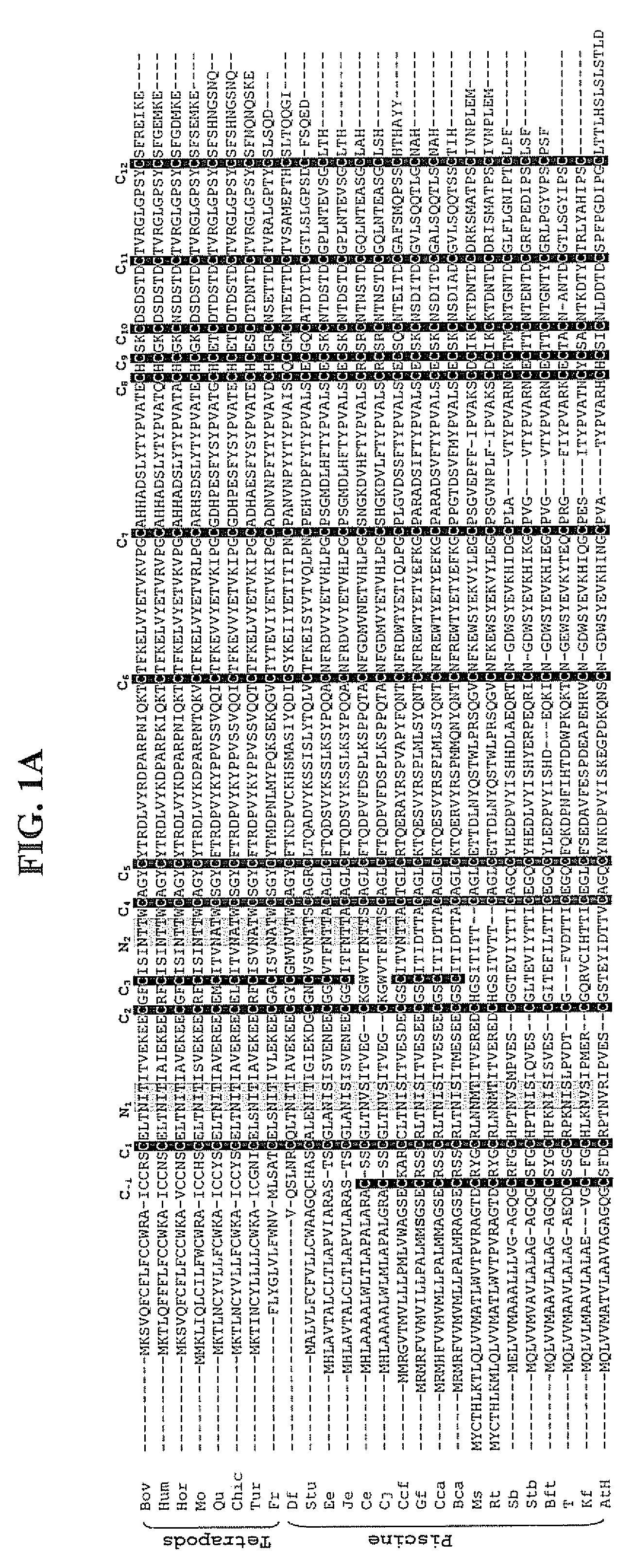
InactiveUS20070281883A1Improve metabolic activityImprove stabilitySugar derivativesPeptide/protein ingredientsBiologyDisulfide bond
The invention provides recombinant forms of piscine follicle-stimulating hormone (FSH) with characteristic intramolecular disulfide bonds and modified glycosylation patterns in the β-subunit that enhance the stability and metabolic activity of the hormone. Also provided are recombinant materials to produce the FSH β and glycoprotein α-subunits singly or in combination to obtain complete heterodimeric hormone of regulated glycosylation pattern. The piscine FSH agonists of the invention are therapeutically useful to expedite the onset of puberty in captive fish and to alleviate reproductive dysfunctions in fish. Likewise, the piscine FSH antagonists of the invention will be therapeutically useful to halt gonadal development, thereby contributing to body weight gain of the fish,
Owner:FISHBREED
Compositions and methods including expression and bioactivity of bovine follicle stimulating hormone
ActiveUS20080312151A1Improve reproductive activityImproving superovulationPeptide/protein ingredientsImmunoglobulinsMammalEmbryo
The present invention provides methods of producing biologically active recombinant bFSH and methods of increasing reproduction in mammals, particularly bovine, using recombinant bFSH. Also provided are methods of producing single chain recombinant bFSH. The recombinant bFSH of the present invention increases superovulation, embryo development, and reproductive efficiency in cattle and other ungulates.
Owner:ASPENBIO PHARMA
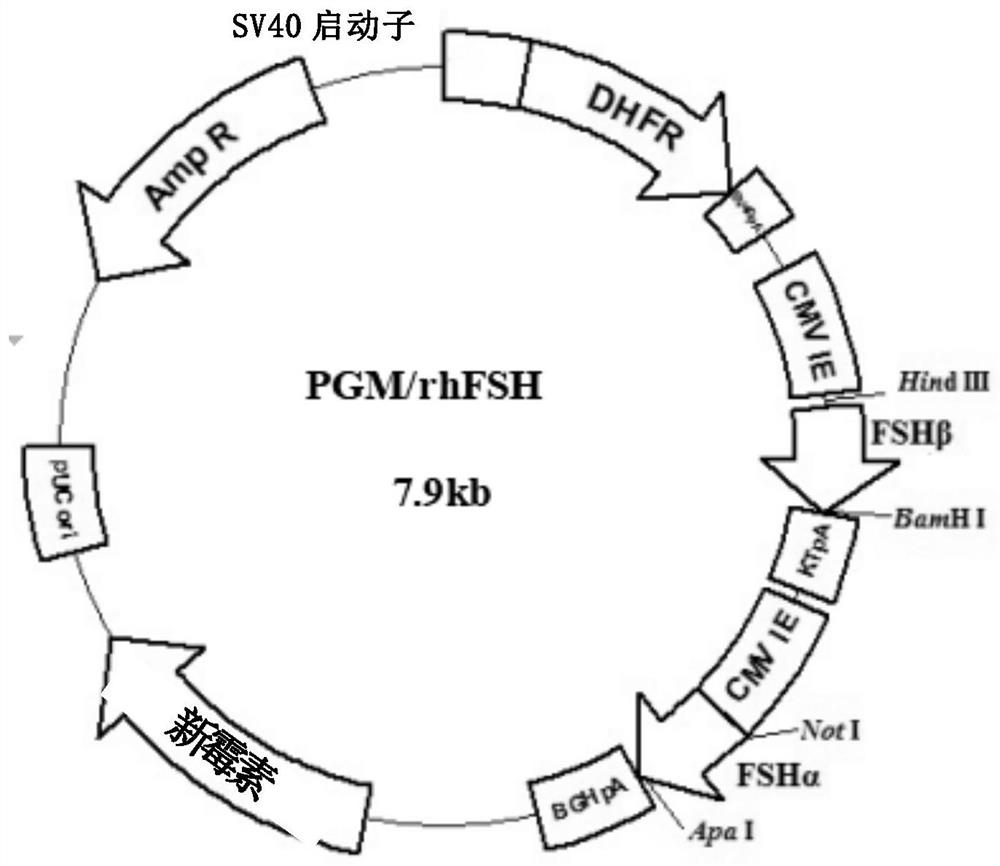
Recombinant human follicle-stimulating hormone and its preparation
ActiveCN102295695AHigh activityHigh expressionPeptide/protein ingredientsDepsipeptidesRecombinant human follicle stimulating hormoneHigh specific activity
The invention provides high-specific-activity recombinant human follicle stimulating hormone and a preparation method thereof. In addition, the invention also provides a method for preparing an intermediate used in the method, gene, vector and cells.
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
Long-acting recombinant follicle-stimulating hormone and application thereof
ActiveCN103554268AProlong half-life in vivoElongated stabilizing proteinPeptide/protein ingredientsPeptide preparation methodsAnimal breedingHalf-life
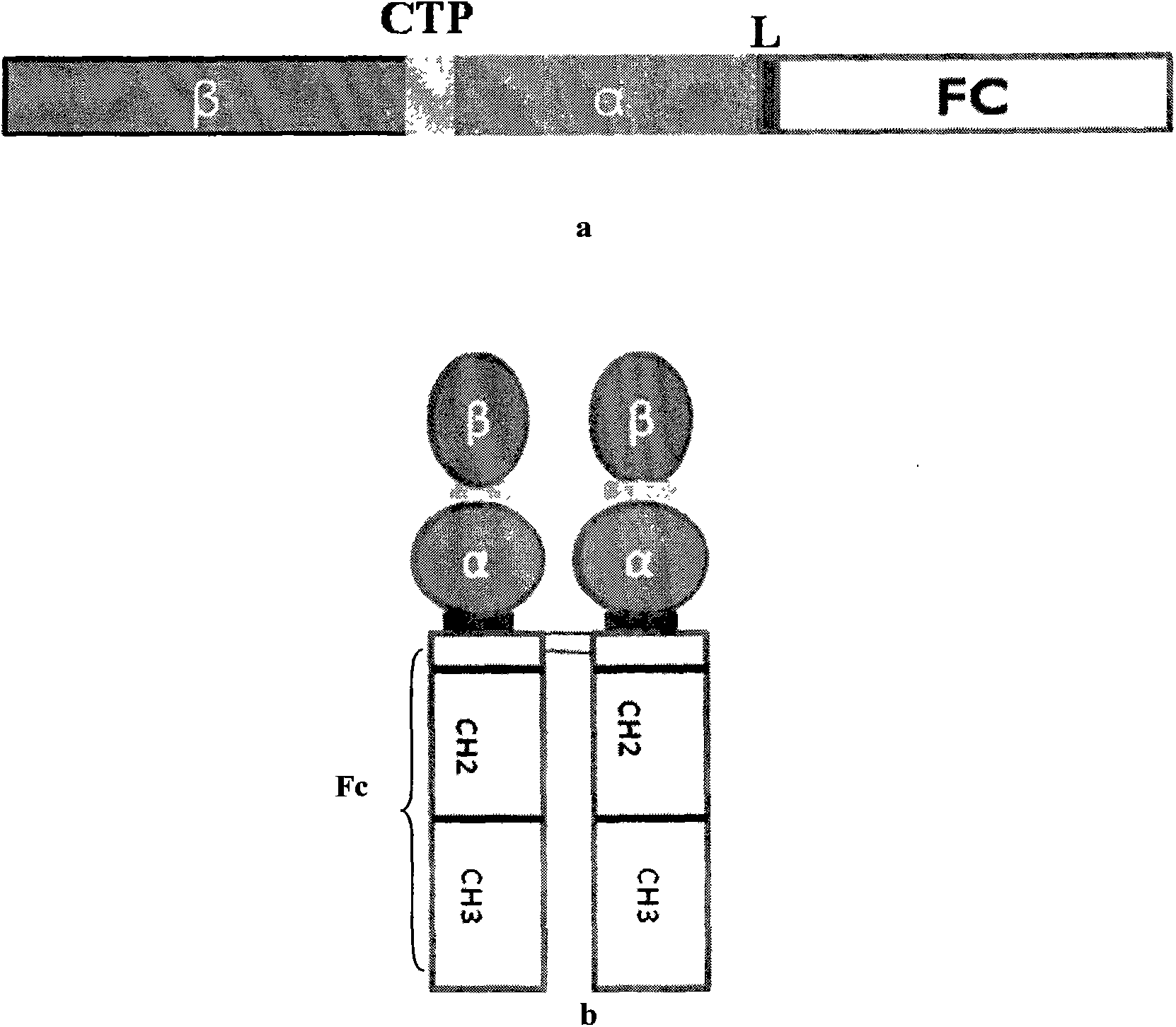
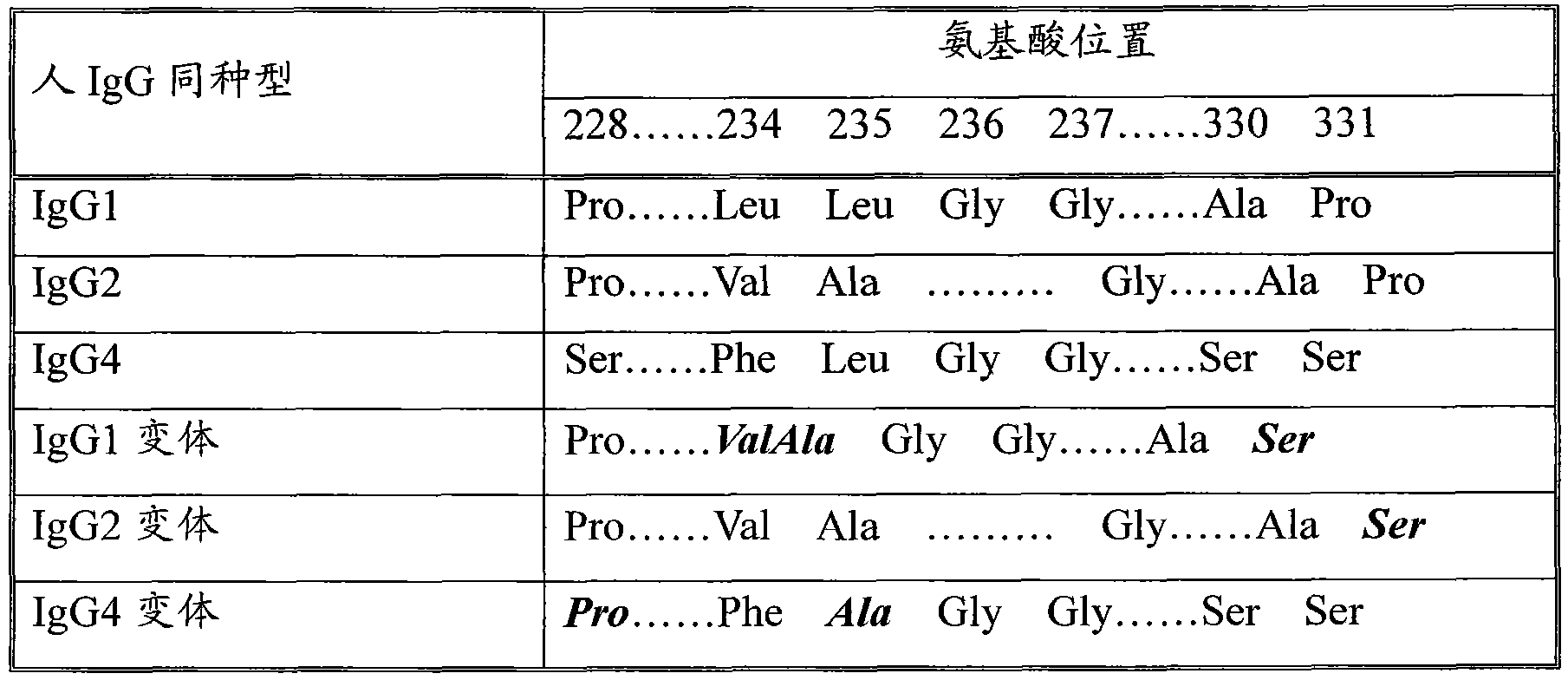
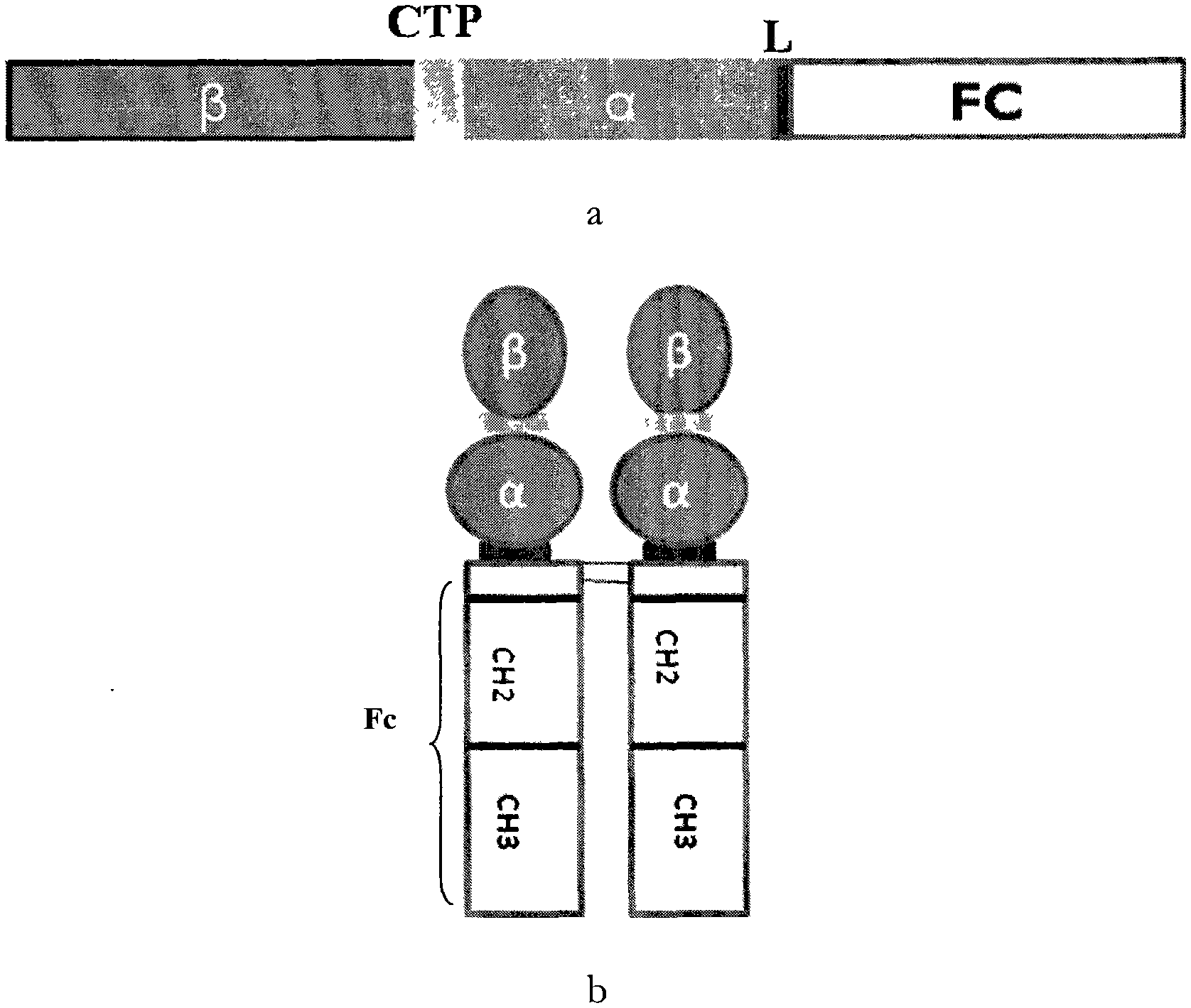
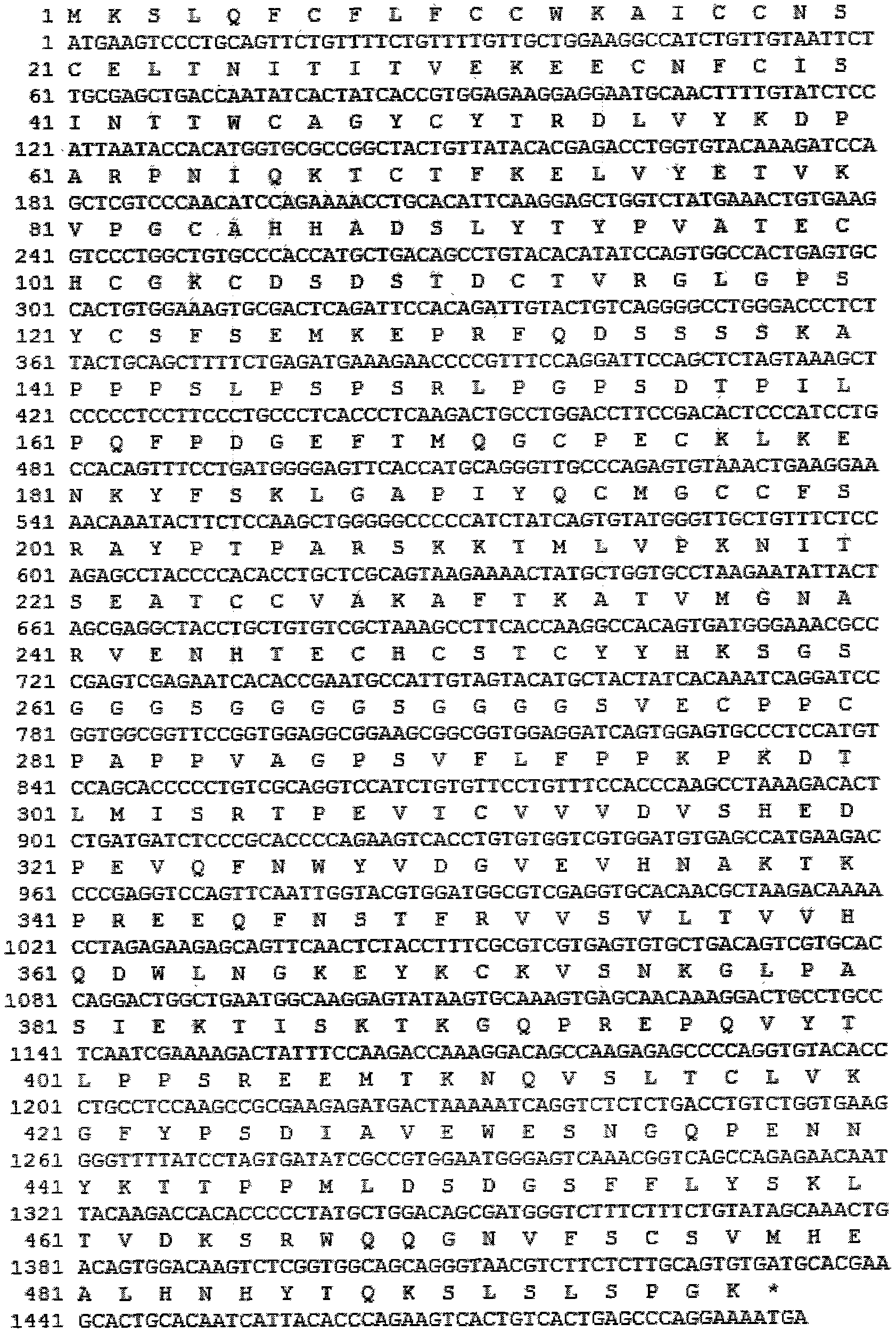
The invention discloses a long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (referred to as hFSH-Fc) and a preparation method thereof. The hFSH-Fc protein is dimerization fusion protein, the amino acid sequence of the protein sequentially comprises an hFSH beta subunit, CTP, a hFSH alpha subunit, a flexible peptide joint and a human IgG Fc variant from the N end to the C end, and the in vivo half-life period of the protein is longer than that of the existing human follicle-stimulating hormone, so that the efficacy is better. The invention also relates to a use of a recombinant hFSH-Fc fusion protein composition in preparation of drugs in the animal breeding field.
Owner:GUANGZHOU VBIO PHARM CO LTD
Recombinant porcine follicle-stimulating hormone-Fc fusion protein (pFSH-Fc)
ActiveCN103554269AProlong half-life in vivoImprove mechanical propertiesPeptide/protein ingredientsPeptide preparation methodsAmino acidOvarian follicle
The invention discloses a recombinant porcine follicle-stimulating hormone-Fc fusion protein (pFSH-Fc) and a preparation method thereof. The fusion protein is a dimerized fusion protein, wherein the amino acid sequence of the fusion protein sequentially comprises pFSH beta subunits, carboxy-terminal peptide (CTP), pFSH alpha subunits, peptide connectors and human IgG Fc variants from a terminal N to a terminal C. Compared with existing pFSH, the pFSH-Fc has a longer in-vivo half-life period and better efficacies. The invention further relates to application of the pFSH-Fc to preparation of medicaments in the animal breeding fields.
Owner:GUANGZHOU VBIO PHARM CO LTD
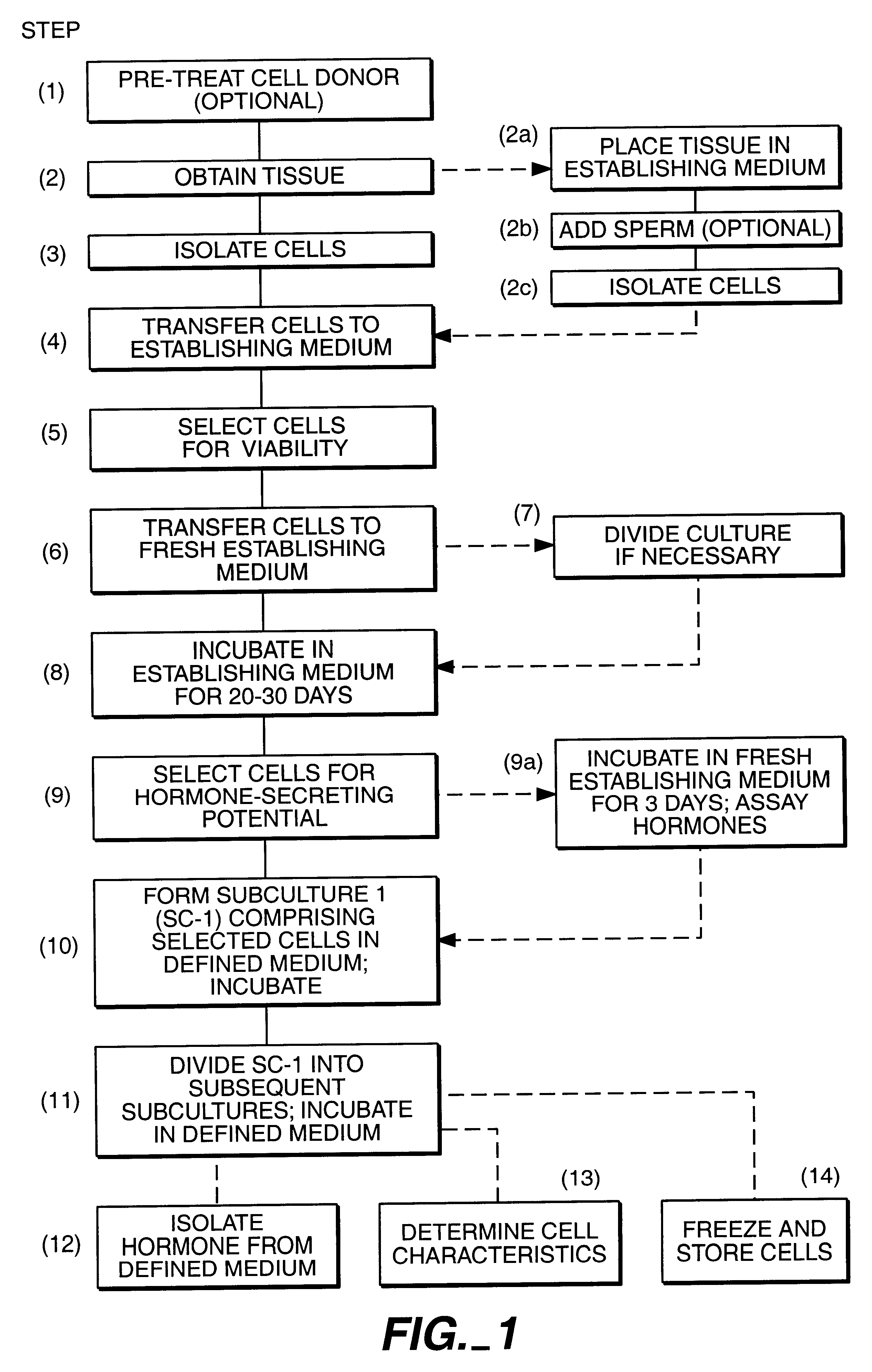
Hormone-secreting cells maintained in long-term culture
InactiveUS6372493B1Increased insulin secretionHigh glucose concentrationPeptide/protein ingredientsDiagnosticsCulture mediumsHuman chorionic gonadotropin
Methods are provided for the establishment and maintenance in long term culture of hormone secreting cells. Cells are derived from tumorous or non-tumorous animal or human tissues, including ovary, endometrium, trophoblast, pituitary, thyroid, and pancreas. The cells secrete into the culture medium hormones such as estrogens, progestins, follicle-stimulating hormone, luteinizing hormone, human chorionic gonadotrophin, thyroxin, glucagon, and insulin, depending on the tissue of origin of individual cell cultures. Contact with an appropriate secretogogue causes the cells to respond with increased hormone secretion. For instance, ovarian follicular cells respond to follicle-stimulating hormone with increased estrogen and progesterone secretion. Pancreatic cells respond to elevated glucose with increased insulin secretion. The cells proliferate in in vitro for up to one year or longer, during which time they retain their hormone-secretion profile. The cells may be frozen for storage, and retain their hormone-secretion profile after thawing. The cell cultures are useful for the production of human hormones, for the bio-assay of drugs such as therapeutic gonadotrophin, for the testing of drug efficacy and design, and for toxicity testing of drugs and chemicals. The cells may also be implanted in an individual to replace deficient hormone secretion. For instance, insulin secreting pancreatic cells may be implanted in a diabetic individual as an adjunct or replacement therapy for exogenously administered insulin.
Owner:PACIFIC BIOMEDICAL RES INC
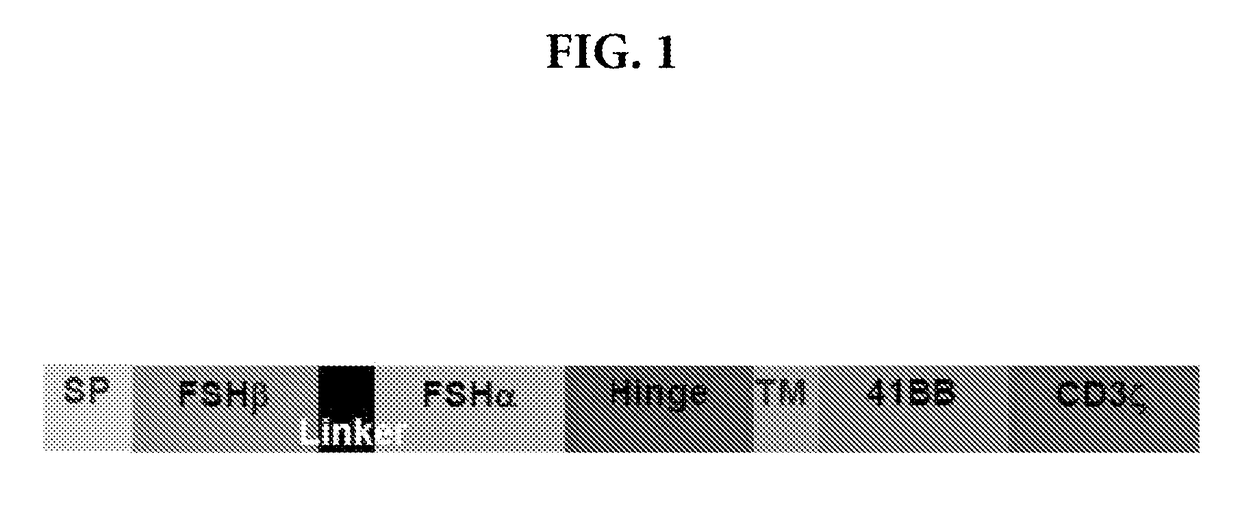
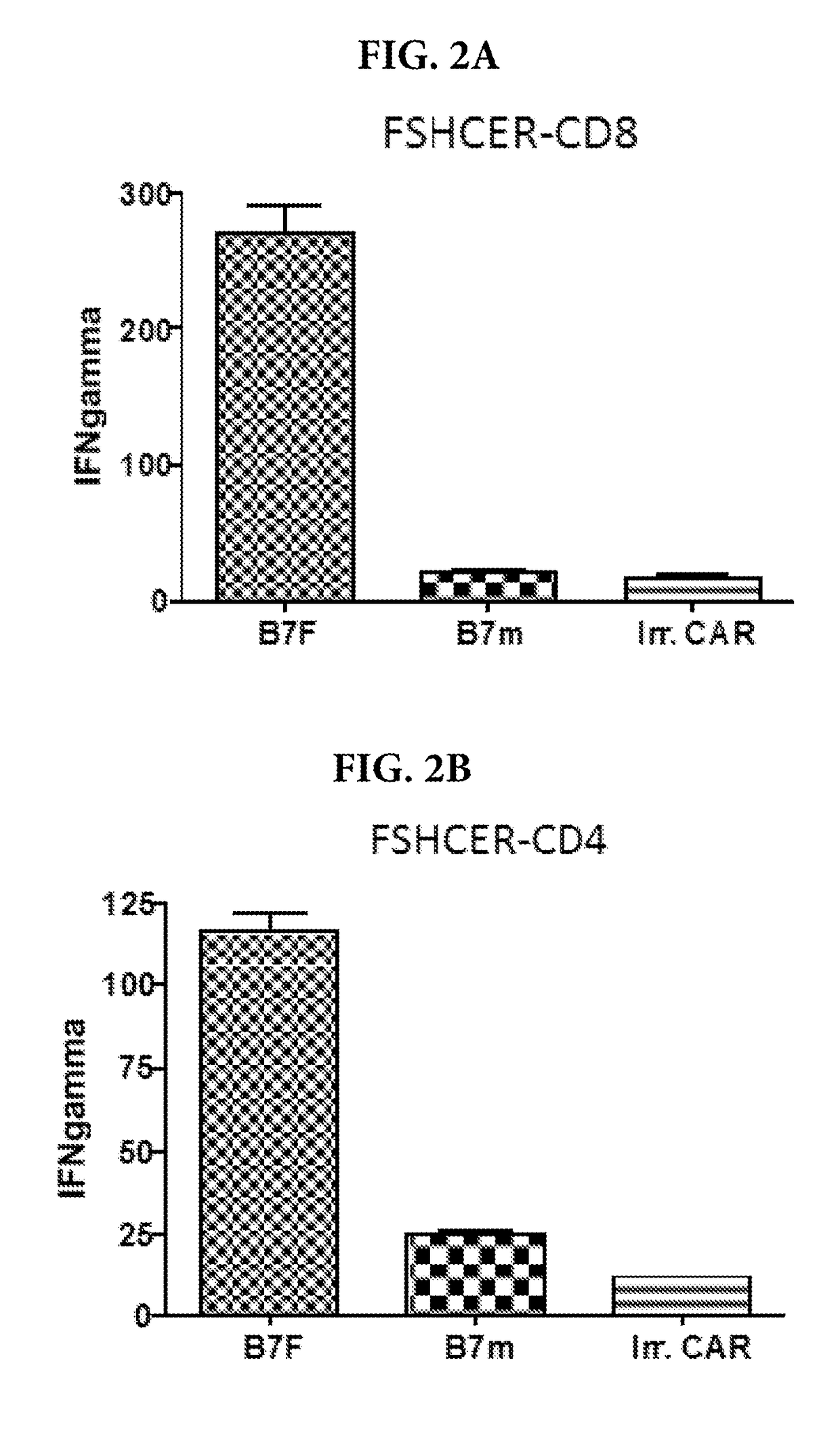
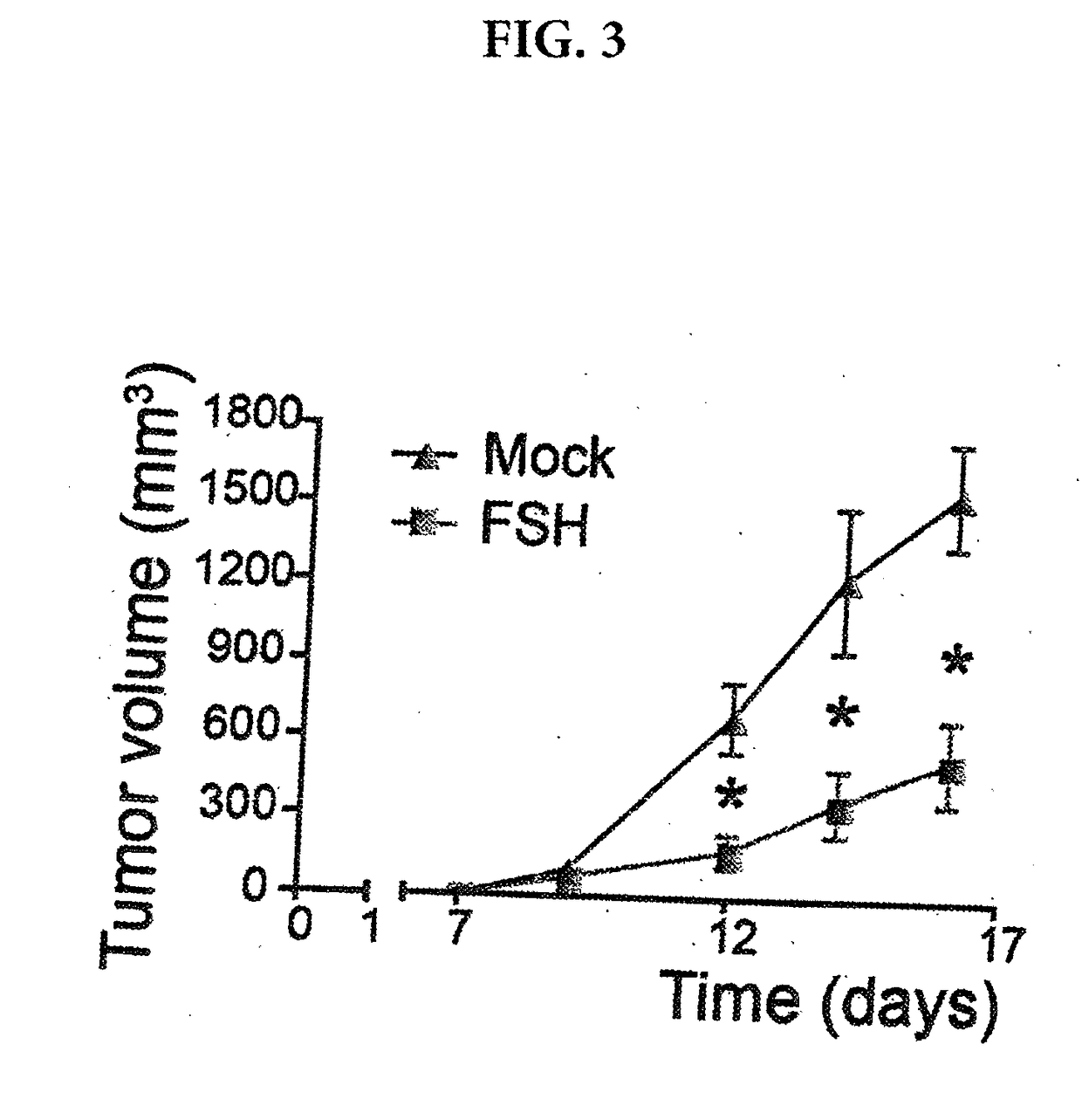
Methods and compositions for treating cancer
ActiveUS20170226176A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyChimera ProteinNucleic acid sequencing
A nucleic acid sequence is provided that encodes a chimeric protein comprising a ligand that comprises a naturally occurring or modified follicle stimulating hormone sequence, e.g., an FSHp sequence, or fragment thereof, which ligand binds to human follicle stimulating hormone (FSH) receptor, linked to either (a) a nucleic acid sequence that encodes an extracellular hinge domain, a transmembrane domain, a co-stimulatory signaling region, and a signaling endodomain; or (b) a nucleic acid sequence that encodes a ligand that binds to NKG2D. The vector containing the nucleic acid sequence, the chimeric proteins so encoded, and modified T cells expressing the chimeric protein, as well as method of using these compositions for the treatment of FSHR-expressing cancers or tumor cells are also provided.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
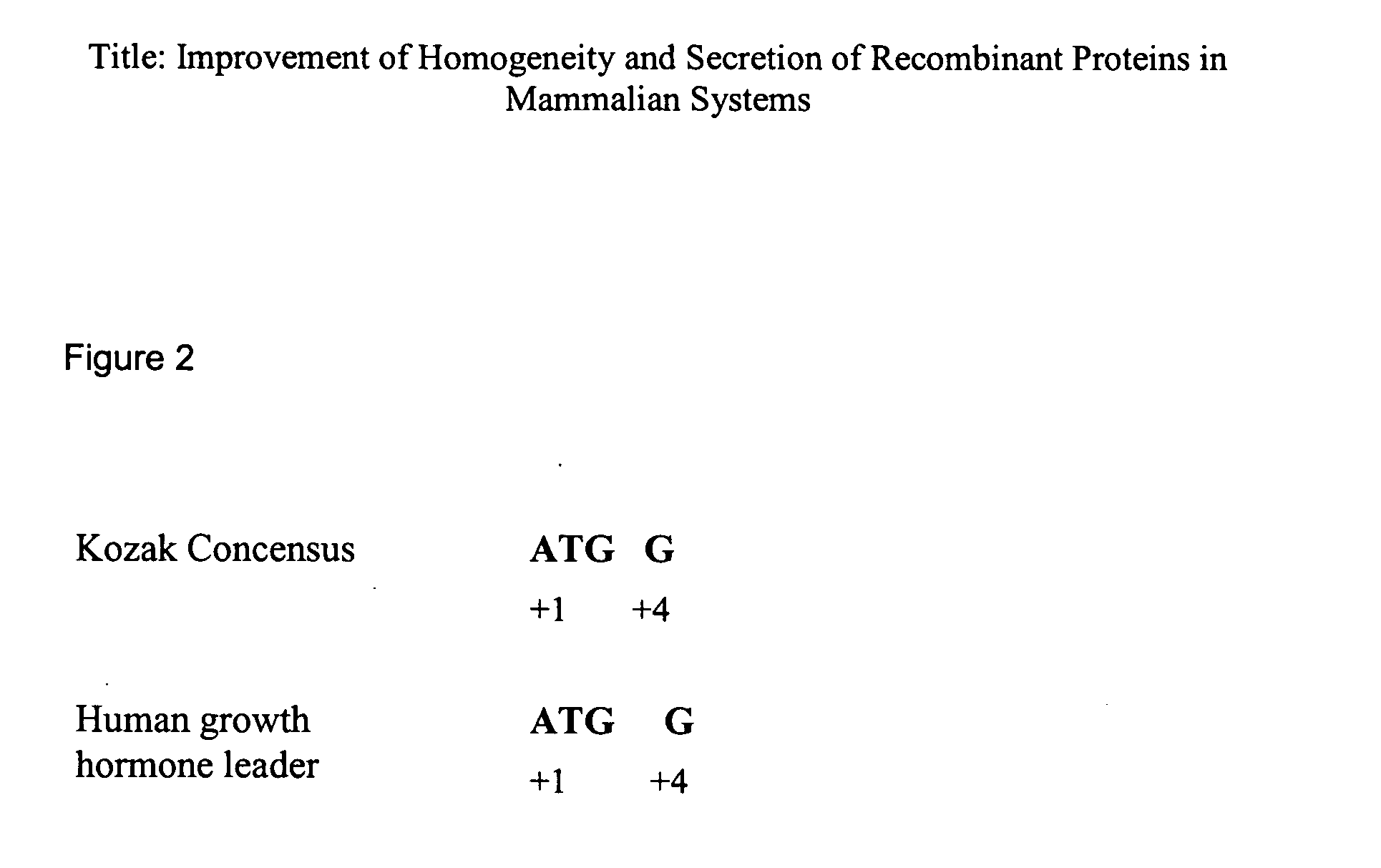
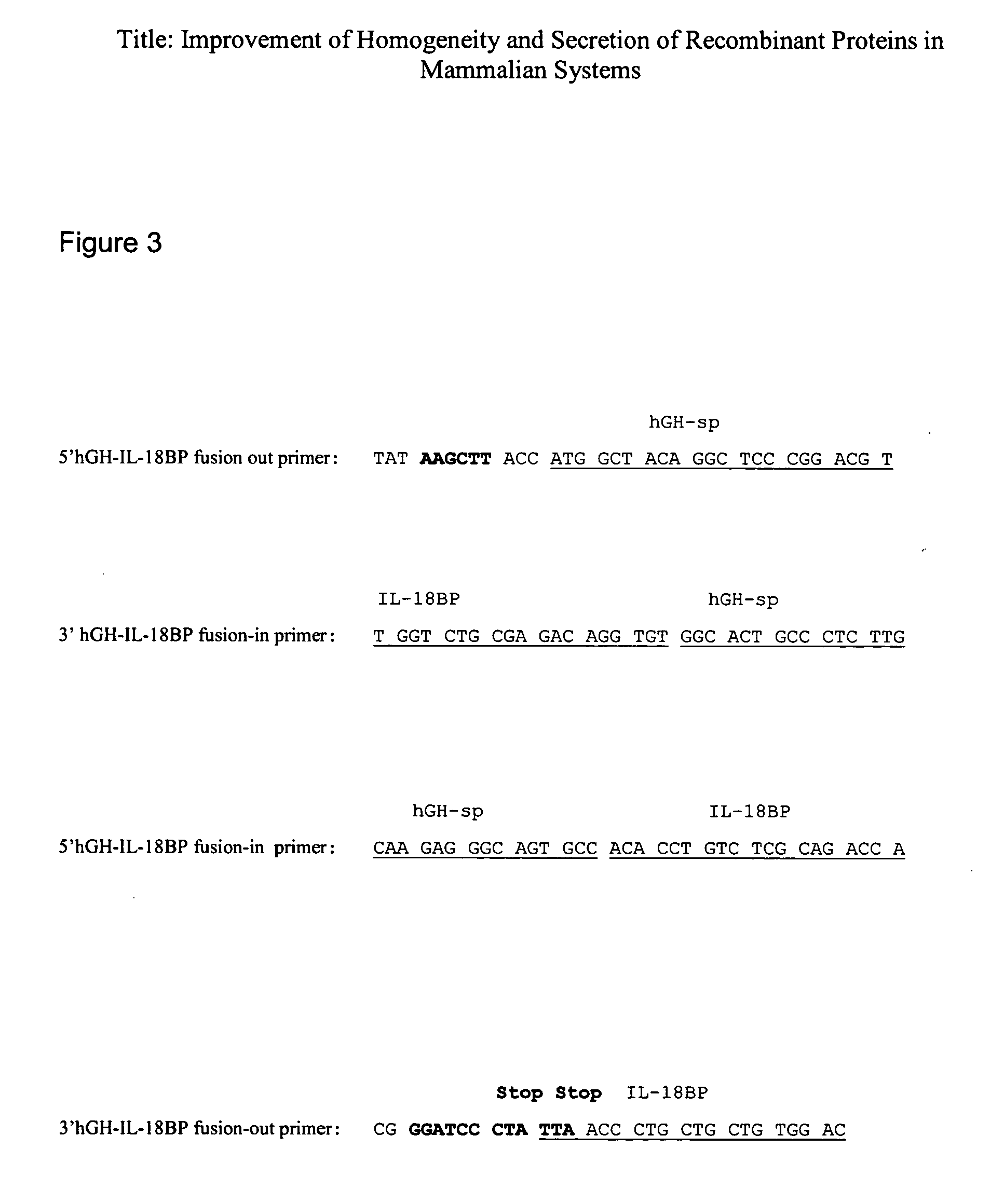
Homogeneity and secretion of recombinant proteins in mammalian systems
InactiveUS20060234352A1Improve homogeneityIncrease secretionPeptide/protein ingredientsAntibody mimetics/scaffoldsMammalFollitropin
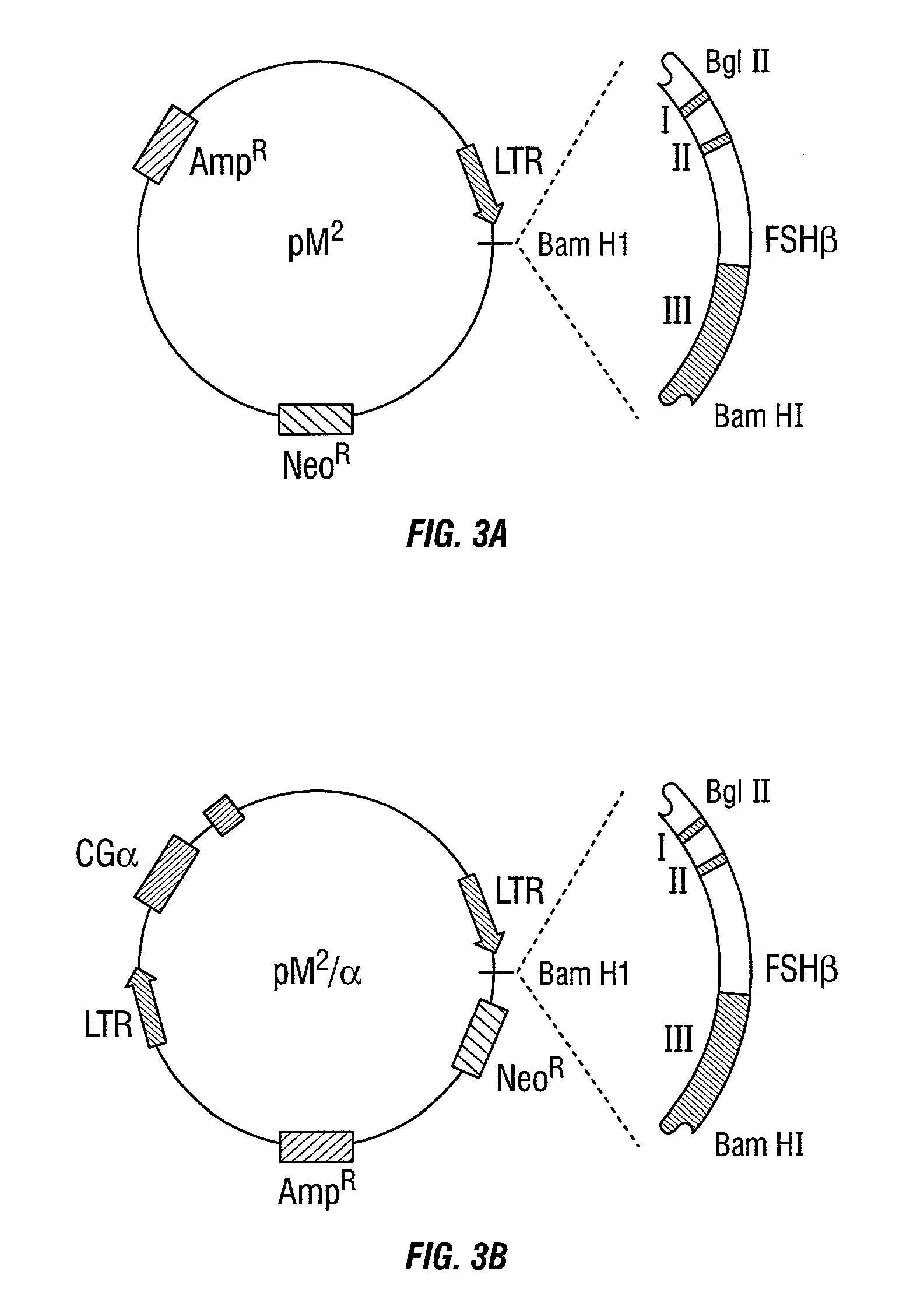
The present invention relates to a method for improving homogeneity and / or secretion of a recombinant protein of interest expressed in mammalian cells by replacing the endogenous signal peptide sequence of the DNA encoding the protein of interest with that of human hGH. Specifically, the present invention relates to a method wherein the protein of interest is a subunit of the follicle stimulating hormone (FSH). The invention also relates to DNA expression vectors containing the sequence encoding such proteins of interest fused to the signal peptide sequence of the hGH and to cells harbouring such vectors.
Owner:MERCK SERONO SA
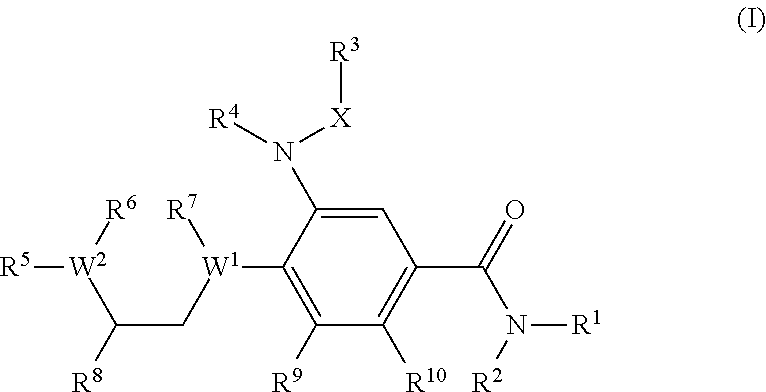
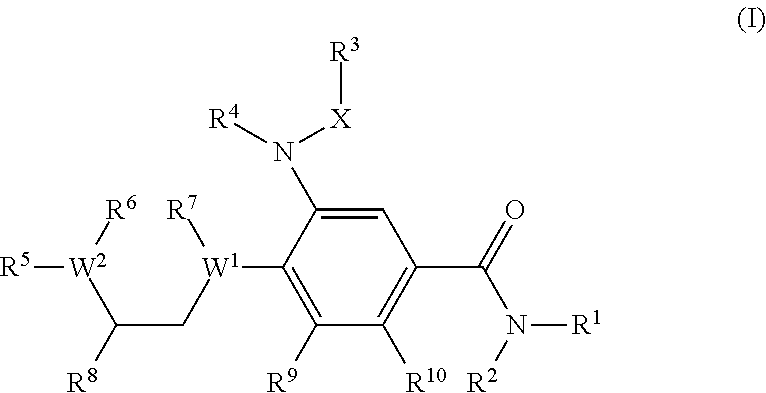
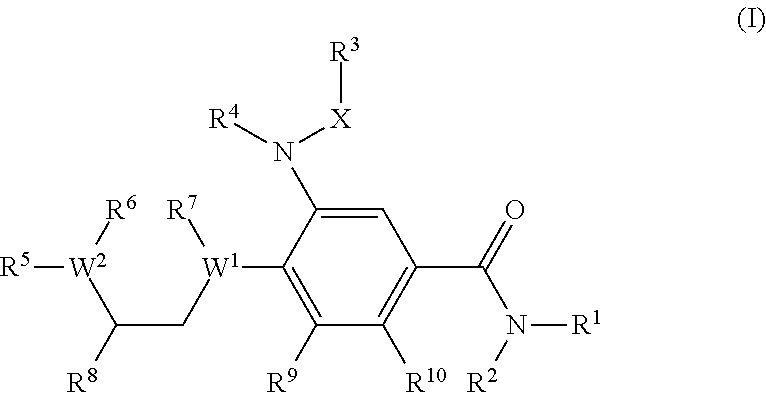
Benzamide derivatives as modulators of the follicle stimulating hormone
ActiveUS20150011562A1Low costReduce usageOrganic active ingredientsAnimal reproductionReceptorAllosteric modulator
Owner:MERCK PATENT GMBH
Long-acting human follicle-stimulating hormone formulation using immunoglobulin fragment
ActiveUS20130022626A1Improve the level ofExtended half-lifePeptide/protein ingredientsAntibody ingredientsSerum igeHalf-life
The present invention relates to a long-acting human follicle-stimulating hormone formulation having improved in vivo duration and stability, comprising a human follicle-stimulating hormone conjugate that is prepared by covalently linking human follicle-stimulating hormone with an immunoglobulin Fc region via a non-peptidyl polymer, and a preparation method thereof. The long-acting human follicle-stimulating hormone formulation of the present invention maintains in vivo activity of human follicle-stimulating hormone at a relatively high level and remarkably increases the serum half-life thereof.
Owner:HANMI SCI CO LTD
Recombinant human follicle-stimulating hormone and preparation method and drug application thereof
PendingCN111349154AIncrease productionSimple processPeptide preparation methodsDepsipeptidesDimerMedicine
The present invention relates to recombinant human follicle-stimulating hormone and a preparation method and a drug application thereof. The recombinant human follicle-stimulating hormone is rhFSH heterodimer containing alpha and beta chains; for a mammalian cell CHO expression system, optimized design of coding genes is performed; and expression plasmids are constructed in a targeted manner, so that expression and correct combination of the alpha and beta chains of the hFSH in mammalian cells CHO are better facilitated. The rhFSH has the advantages of simple process, high target product yield, high purity, high in vivo activity, convenience for intermediate process control, easiness for large-scale production and the like.
Owner:ZONHON BIOPHARMA INST
System for the ovarian reserve function assessment of subjects
PendingUS20220044822A1Quickly and accurately assessPoor repeatabilityUltrasonic/sonic/infrasonic diagnosticsHealth-index calculationObstetricsPhysiology
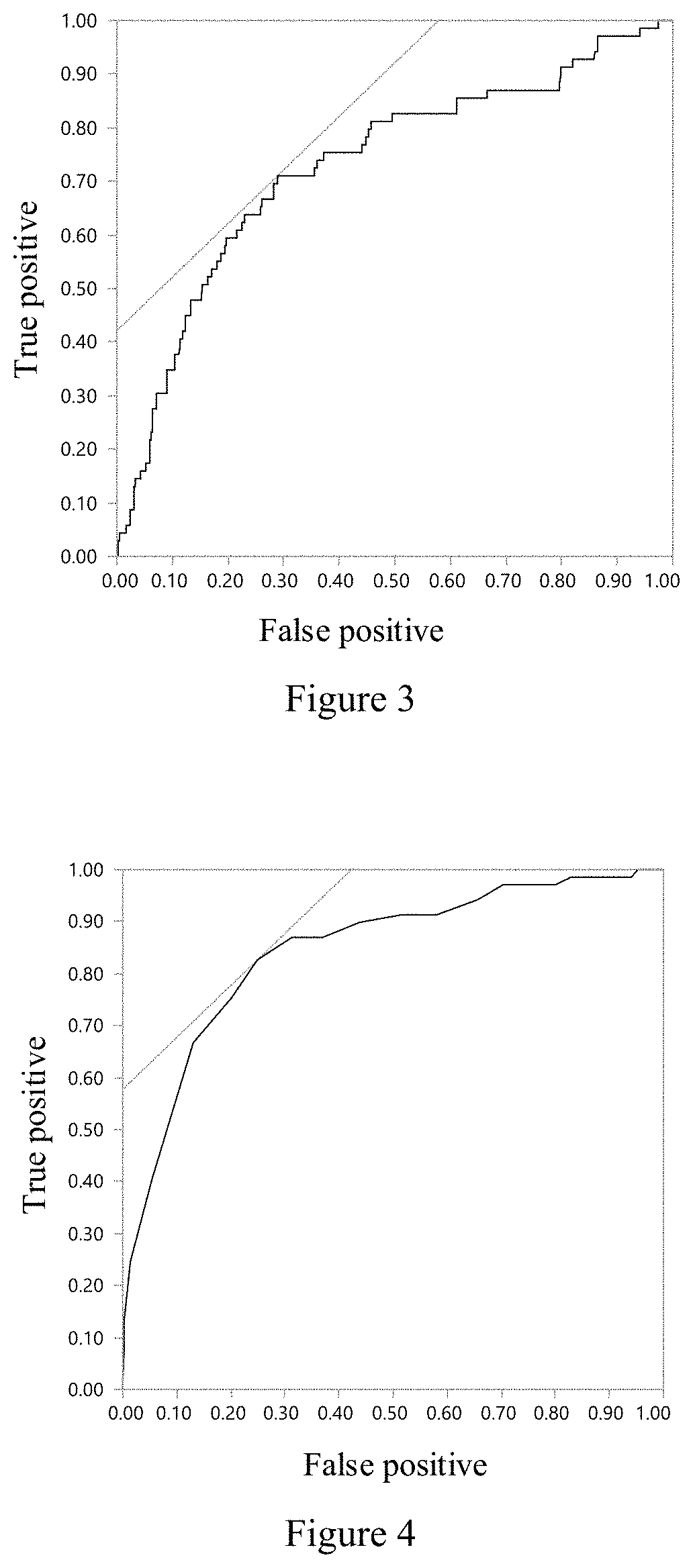
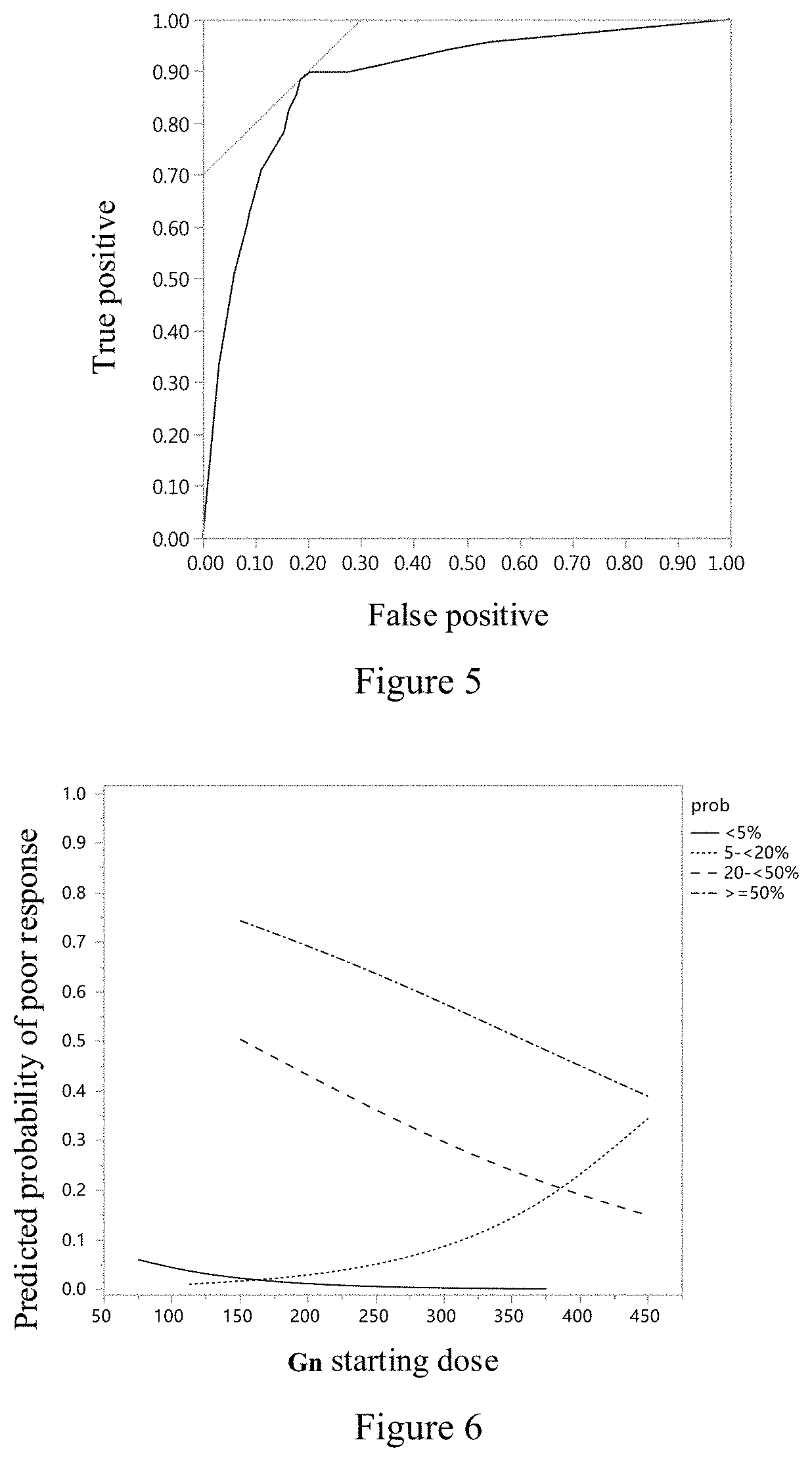
A system and a method for assessing the ovarian reserve function of a subject. The system for assessing a subject's ovarian reserve function comprises: a data acquisition module configured to acquire data of the subject's age, anti-Müllerian hormone (AMH) level, follicle stimulating hormone (FSH) level, and antral follicle count (AFC); and an ovarian reserve function calculation module configured to calculate the above-mentioned information acquired in the data acquisition module, so as to work out the subject's probability of poor ovarian response (p). Further, the population is divided into four groups according to the interaction relationship between the predicted probability of poor ovarian response and the dose of ovulation stimulants, so as to divide the population with similar ovarian responses into one group.
Owner:GUANGZHOU KANGRUN BIOTECHNOLOGY CO LTD
Follicular granular cells near-infrared fluorescent probe Nirova-2 as well as preparation method and application thereof
InactiveCN111053922AEasy to prepareImprove stabilityIn-vivo testing preparationsFluoProbesGranular leucocyte
The invention belongs to the technical field of medical imaging contrast agents, and particularly relates to a follicular granular cells near-infrared fluorescent probe Nirova-2 and a preparation method and application thereof. The fluorescent probe provided by the invention is a fluorescent dye-human follicle stimulating hormone coupling complex formed by coupling an organic small molecule near-infrared dye IR800CW and human follicle stimulating hormone (FSH); according to the complex, the fluorescent dye can specifically target granular cells forming follicle walls in ovaries, so that near-infrared fluorescent labeling of all follicles is realized. The fluorescent probe can be used as a near-infrared region fluorescent contrast agent for the follicles in the ovaries by combining the characteristic of fluorescence emission of the IR800CW in a near-infrared region with the property of specific targeted binding of the FSH to the follicle granular cells in the ovaries, so that the numberand the development stage of the follicles in the ovaries of women can be accurately and effectively detected and evaluated.
Owner:FUDAN UNIV
Recombinant human follicle-stimulating hormone and its preparation method
The invention provides a recombinant human follicle-stimulating hormone and a preparation method thereof. Specifically, steps are included: (1) the first expression cassette for expressing FSH α subunit and the second expression cassette for expressing FSH β subunit are operatively inserted into the eukaryotic expression vector to obtain the protein capable of expressing FSH α subunit and the transfection vector of FSH beta subunit protein; (2) transforming the transfection vector into eukaryotic cells, thereby obtaining the transformed eukaryotic cells integrated with the first expression cassette and the second expression cassette in the chromosome; (3) Under conditions suitable for expression, the transformed eukaryotic cells are cultivated to express the FSHα subunit and the FSHβ subunit, thereby obtaining a fermentation product containing recombinant follicle-stimulating hormone; (4) to the described The fermentation product is separated and purified to obtain the recombinant follicle-stimulating hormone. The method of the invention can replace the use and preparation method of the existing human FSH, and obtain rhFSH with significantly improved activity and purity.
Owner:JINGZE PHARMA (HEFEI) CO LTD +2
Method for determining follicle-stimulating hormone
InactiveCN111044735ALess quantityRapid Quantitative DetectionBiological material analysisBiological testingStainingAntibody conjugate
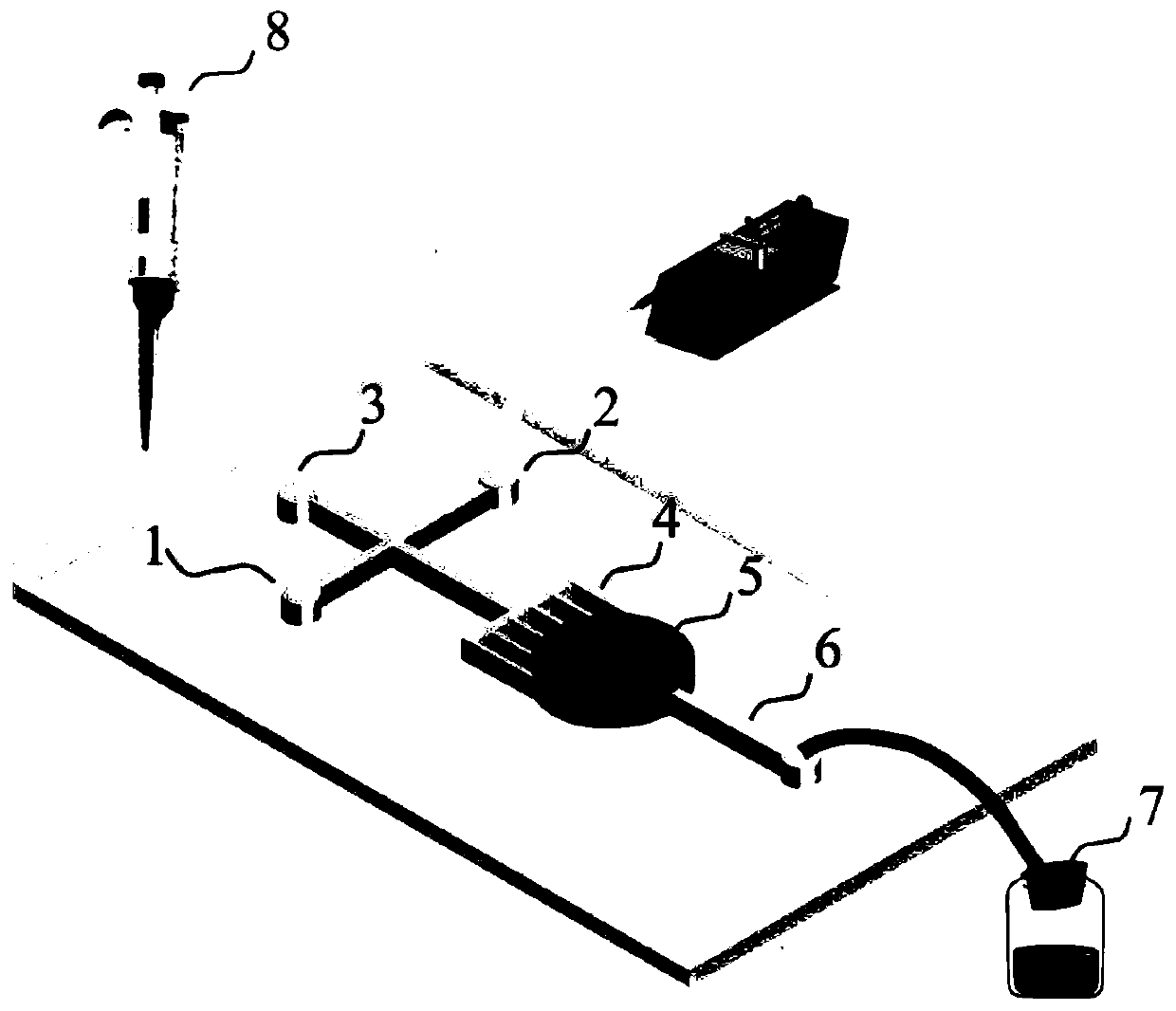
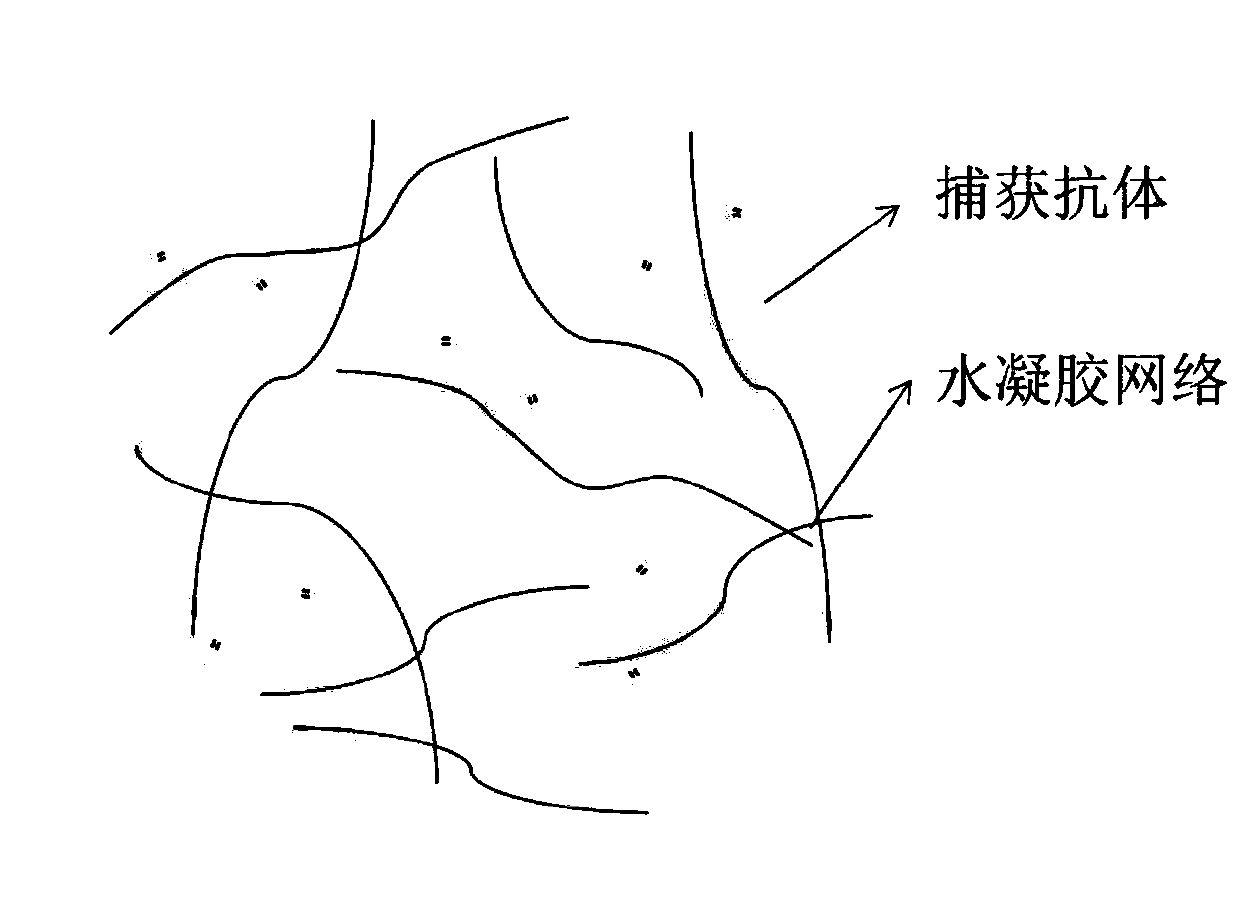
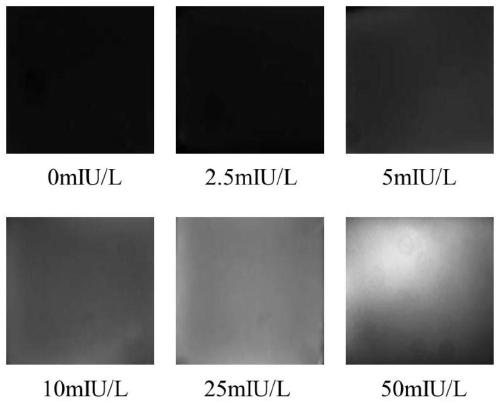
The invention relates to the technical field of biological detection methods, in particular to a method for determining follicle-stimulating hormone. The method comprises the following steps of: (1) manufacturing a micro-fluidic chip; (2) mixing hydrogel with a capture antibody, and curing the mixture in the micro-fluidic chip; (3) adding the micro-fluidic chip into a detection sample and incubating the sample, so that FSH in the detection sample is combined with the capture antibody; (4) adding a dyed detection antibody and incubating the sample, so that the dyed detection antibody is combined with the FSH-antibody conjugate to emit fluorescence; and (5) photographing the detected hydrogel, analyzing the fluorescence intensity of the photographed picture, and calculating the content of follicle-stimulating hormone in the detected sample. According to the method for determining the follicle-stimulating hormone, a novel detection mode is generated through combination of a microfluidic technology and ELISA, the required sample amount is small, and one-step, rapid and quantitative detection of the FSH content in the detection sample is realized.
Owner:WUHAN UNIV
Method for purifying follicle stimulating hormone
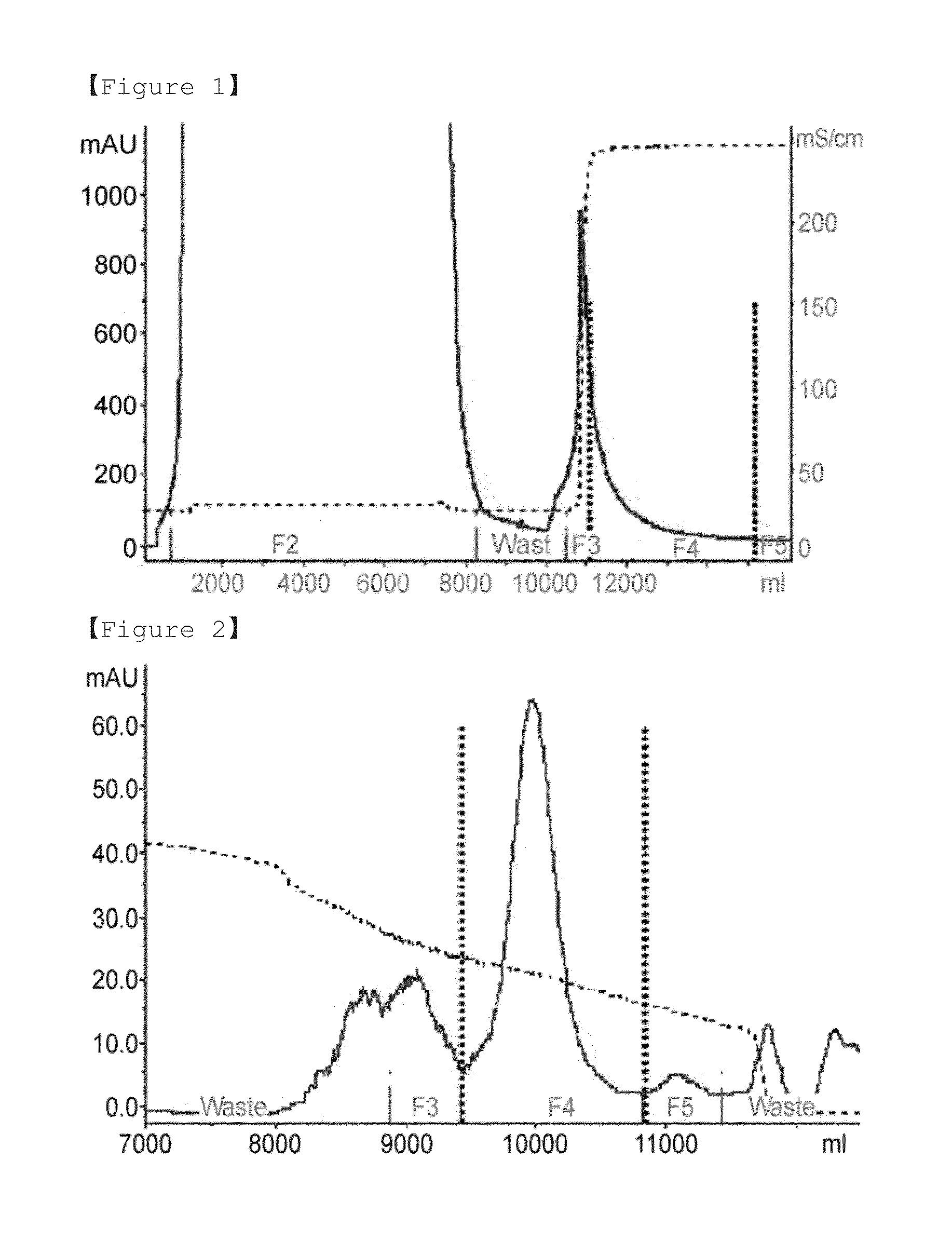
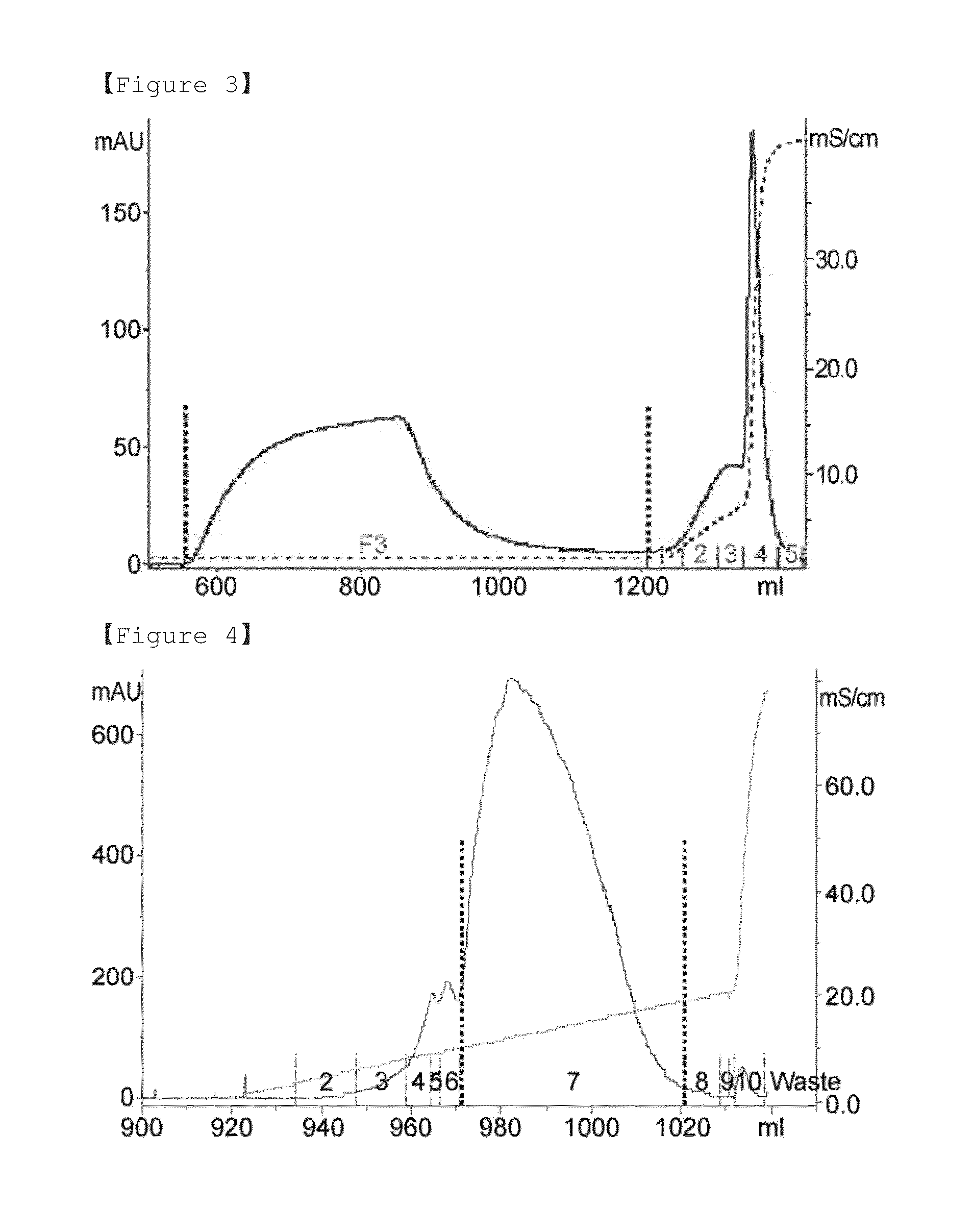
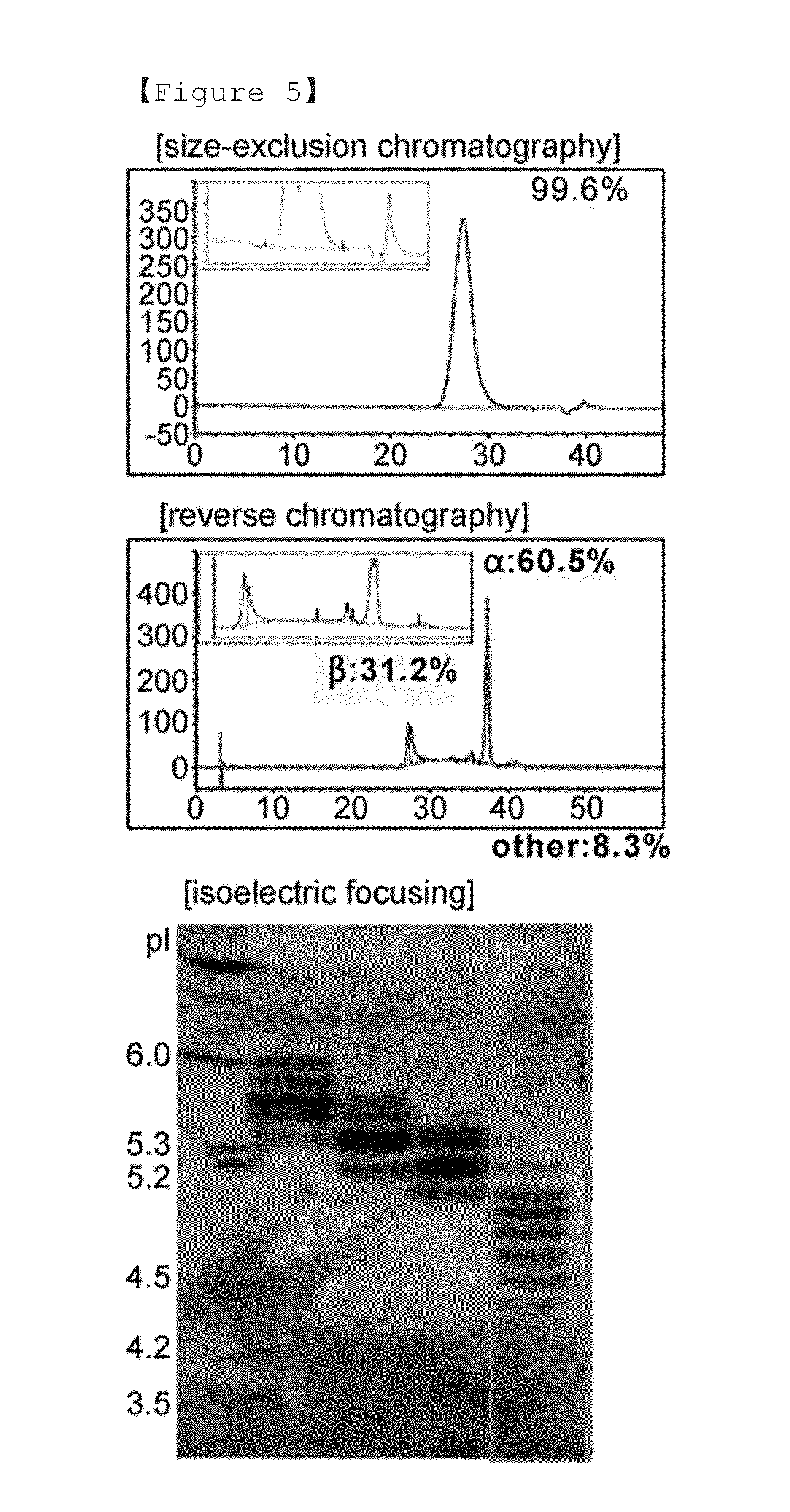
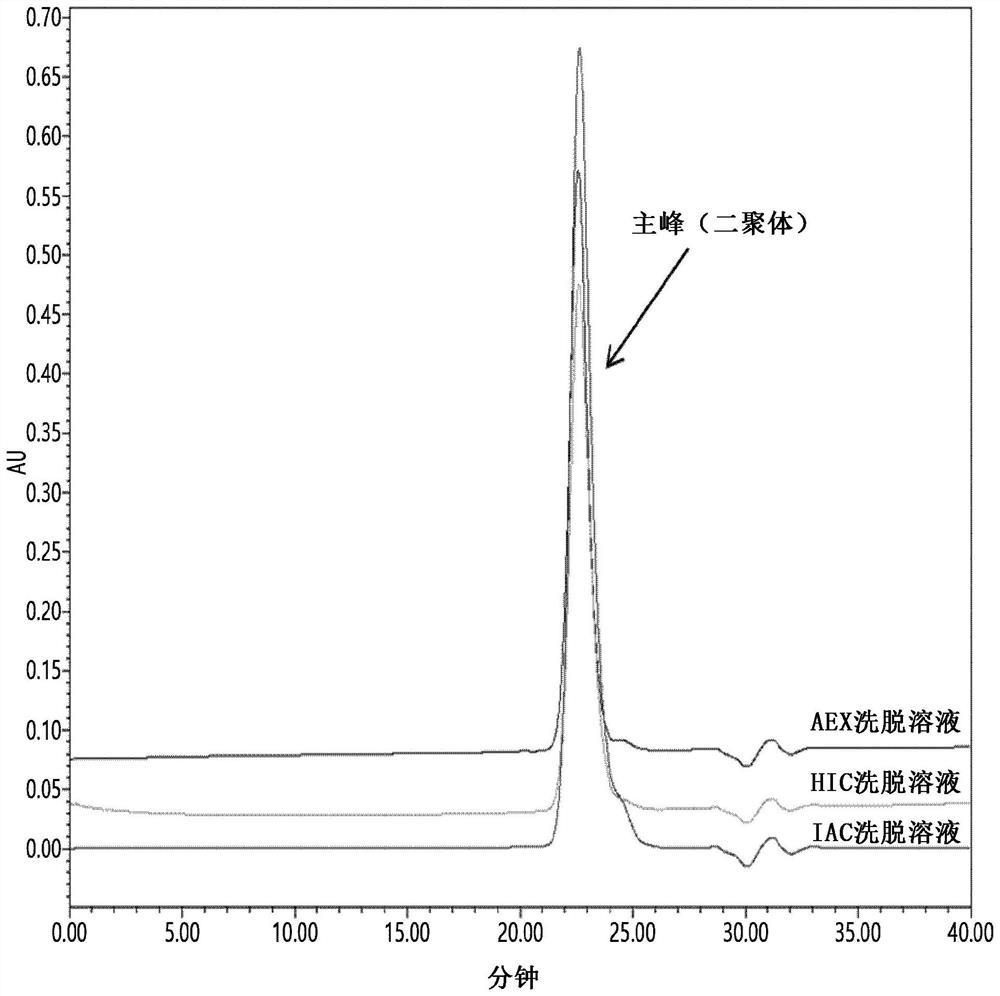
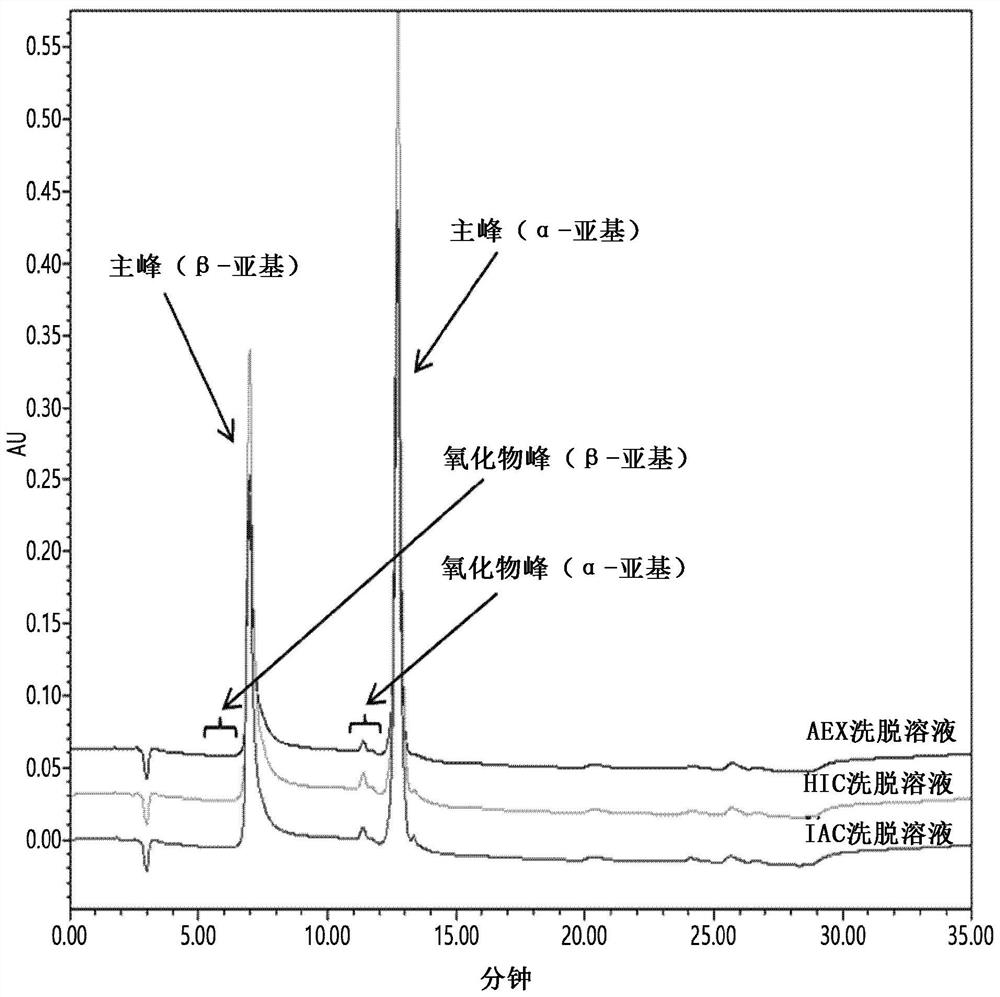
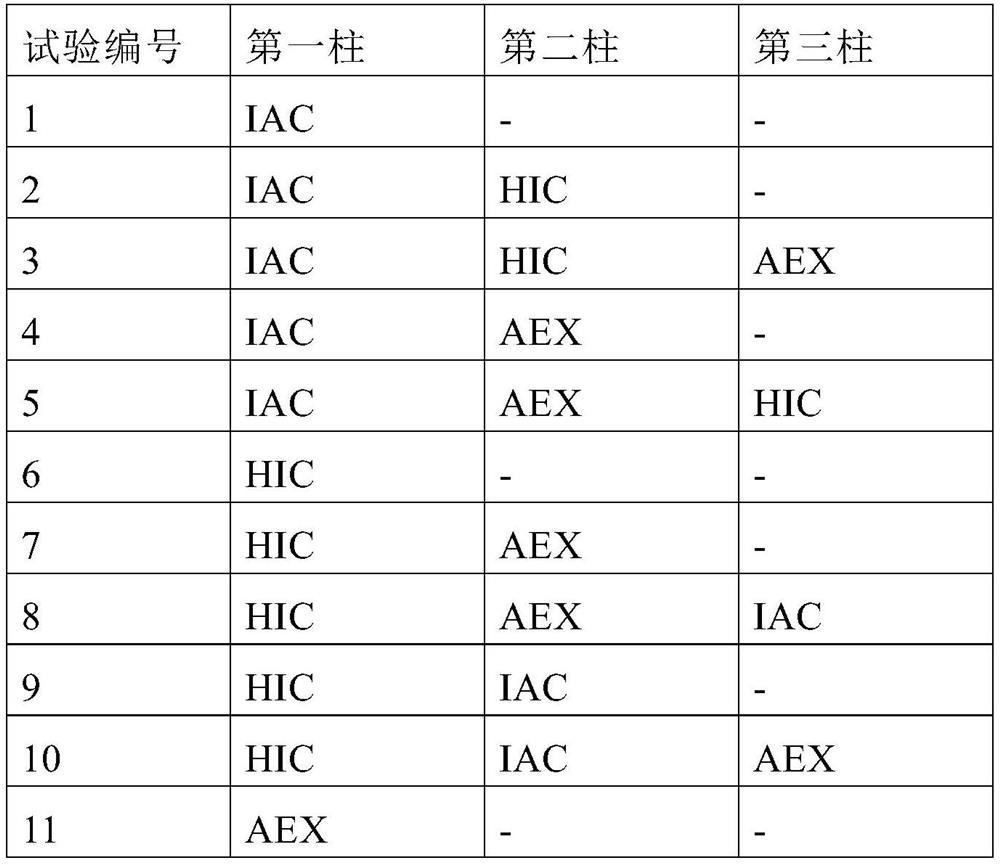
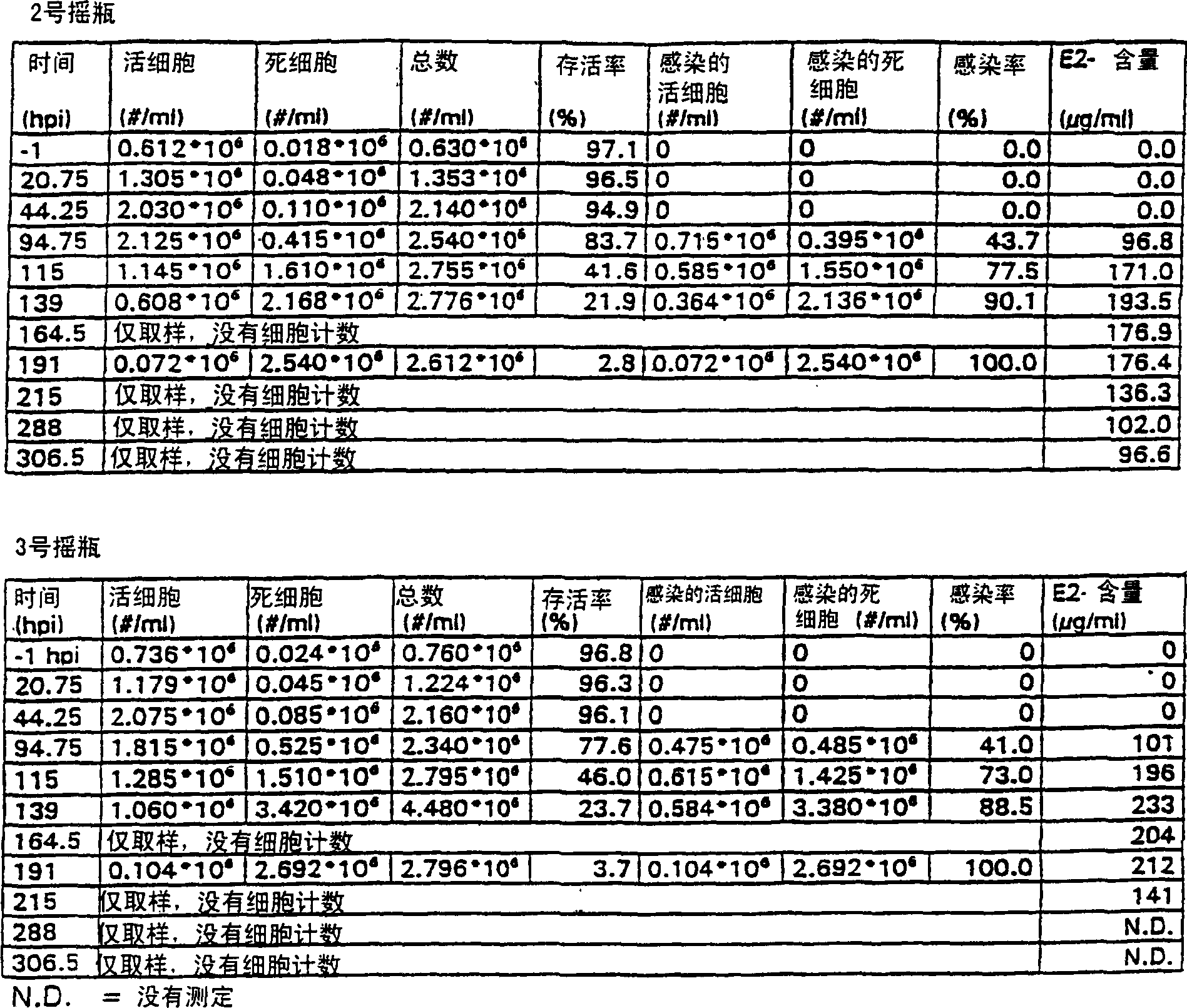
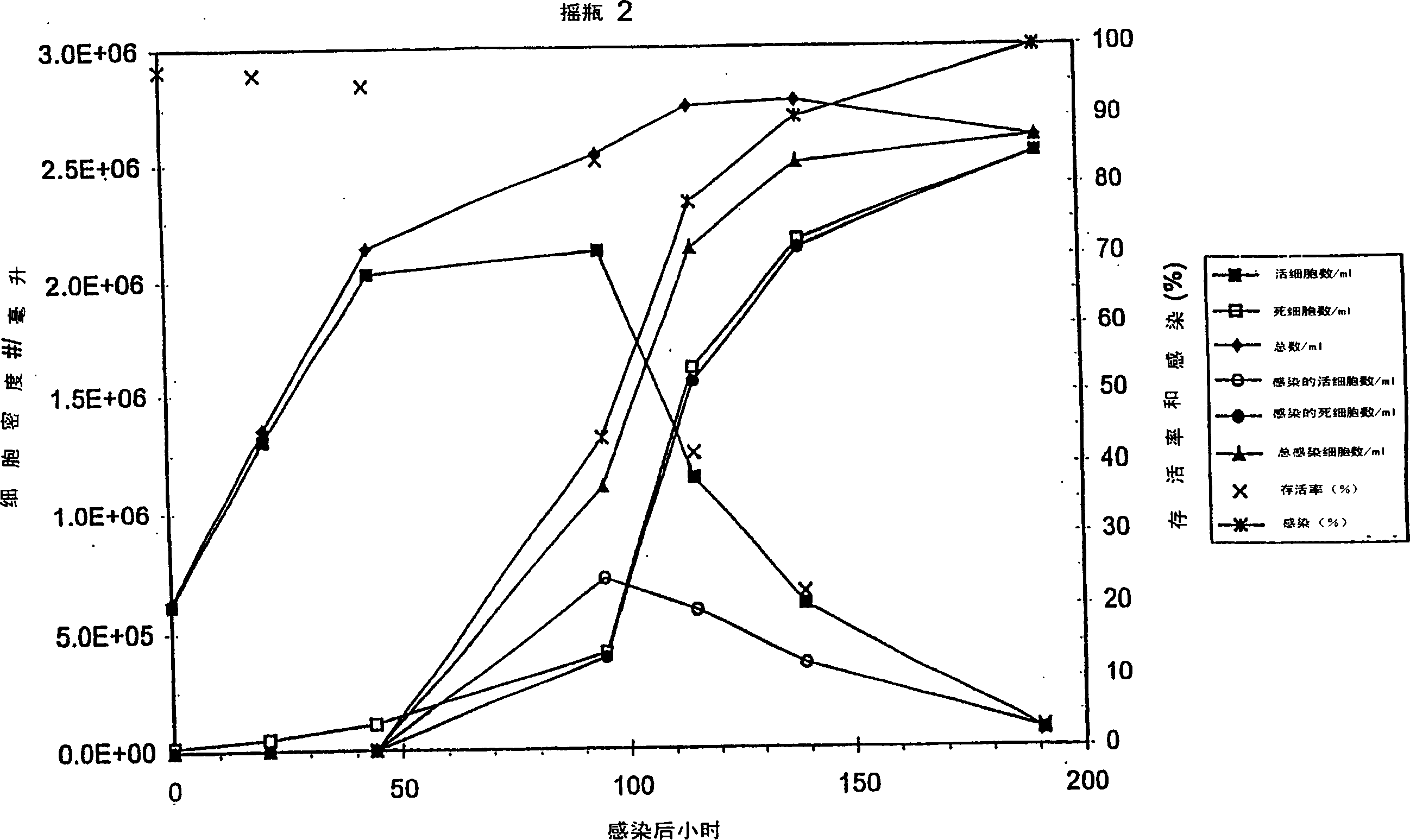
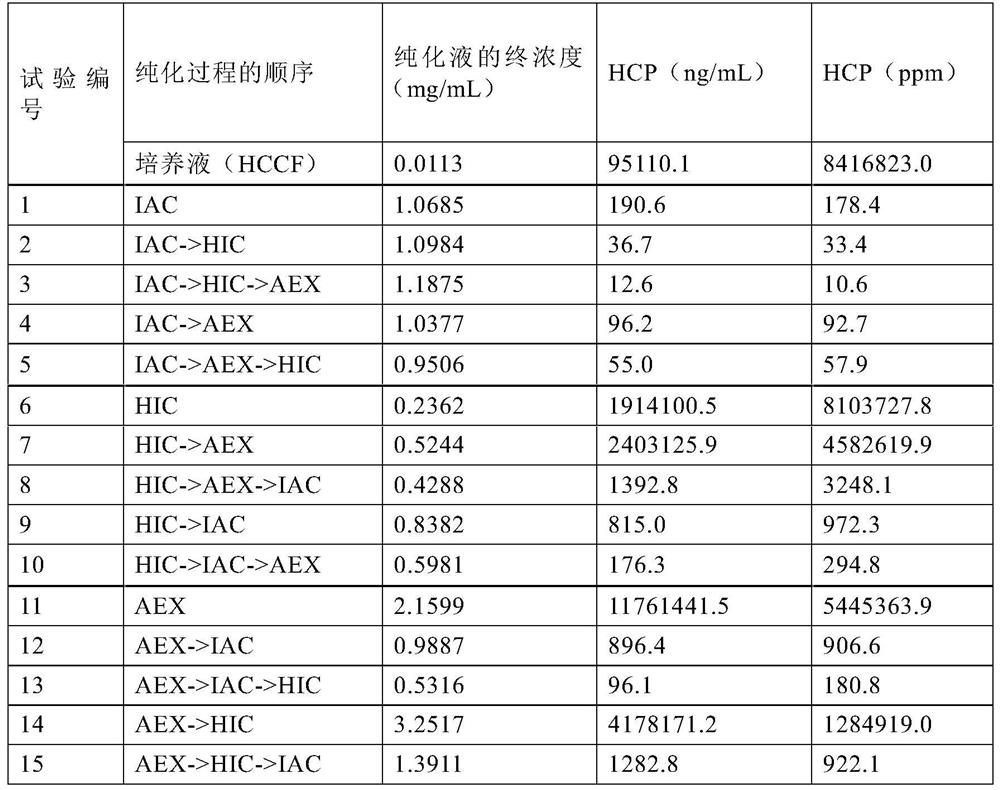
PendingCN114867740AHigh yieldImprove operational efficiencyPeptide preparation methodsDepsipeptidesPhysiologyAffinity chromatography
The present invention relates to a method for purifying follicle stimulating hormone in high yield and high purity, said method comprising an immunoaffinity chromatography (IAC) step.
Owner:LG CHEM LTD
Protein expression in baculovirus vector expression system
InactiveCN1283801CSsRNA viruses positive-senseViral antigen ingredientsInfected cellCell culture media
The present invention relates to methods of enhancing protein expression in baculovirus expression systems. The present invention provides a method for producing a recombinant protein in insect cell culture comprising selecting a recombinant baculovirus expressing said protein, growing insect cells in a sufficient volume of culture vessel containing at least 2 liters of growth medium, and using at least An inoculum of baculovirus infects cells at a density of 1 x 10 at a multiplicity of infection <0.01 5 to 5×10 6 cells / ml of cells. The present invention further provides for the production of recombinant pestiviruses E2 or E in insect cell cultures having a final concentration of said protein (fragment) in the growth medium at harvest of at least 100 μg / ml ms protein or fragment method. The present invention further provides a method for producing recombinant follicle stimulating hormone, its alpha subunit and / or beta subunit or complexes and fragments thereof, at a concentration of 15 micrograms / ml in the growth medium at harvest.
Owner:STICHTING INST VOOR DIERHOUDERIJ & DIERGEZONDHEID +1
Preparation method of recombinant human follicle-stimulating hormone with high specific activity
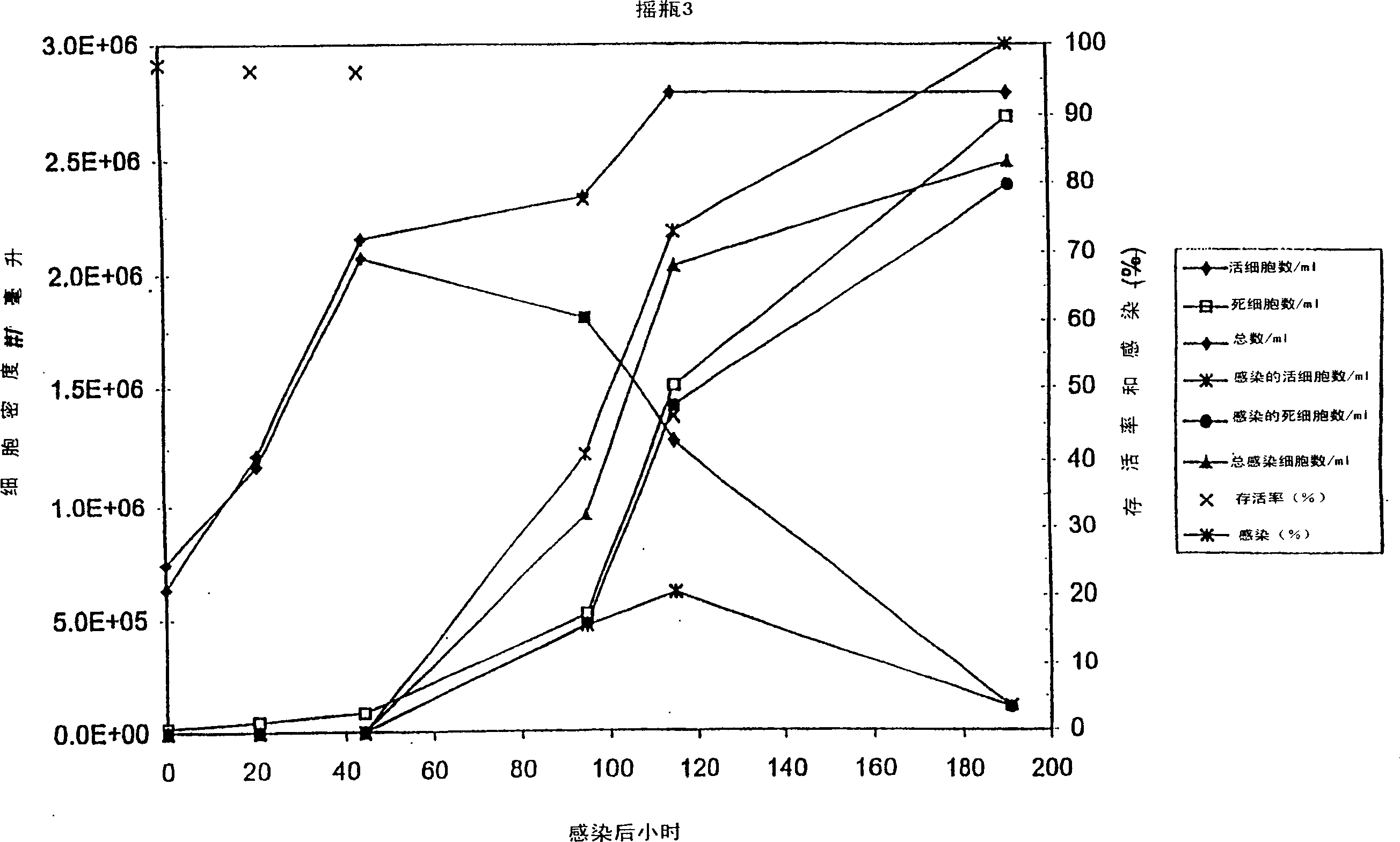
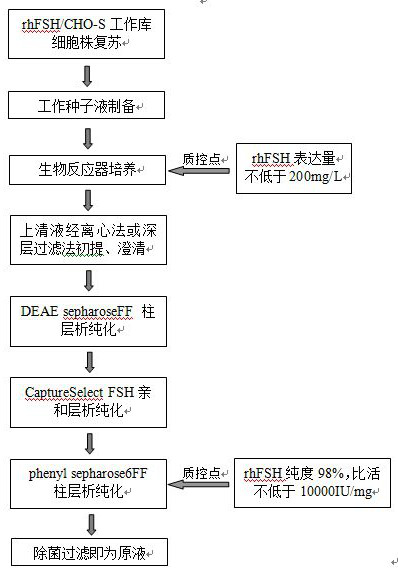
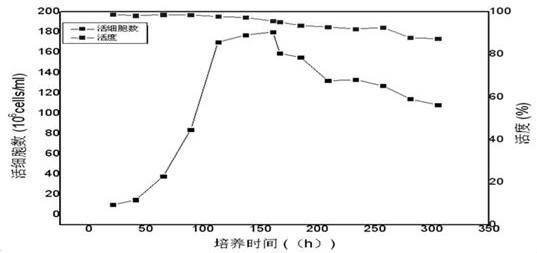
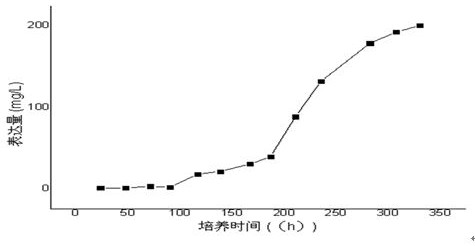
PendingCN112679601AHigh expressionImprove stabilityDepsipeptidesPeptide preparation methodsFollicleCell strain
The invention relates to the technical field of follicle-stimulating hormones, in particular to a preparation method of a recombinant human follicle-stimulating hormone with high specific activity, which comprises the following steps: (1) introducing a cell line of an rhFSH / CHO-S working library into a shake flask filled with a CD Forti CHO culture medium (containing 8mM glutamine), resuscitating, shaking and culturing for 2-4 days, and when the concentration of living cell reaches 2-3*106 / ml and the activity is not less than 80%, transferring to the next stage shake flask according to the density of 2-4*105 / ml, and amplifying step by step according to the same method to complete the preparation of a seed solution; and (2) inoculating the seed solution into a bioreactor according to the density of 4-6*105 / ml of living cells, controlling the dissolved oxygen to be 30-60%, the rotating speed to be 180-220 rpm (7L bioreactor), the rotating speed to be 100-140 rpm (14L bioreactor), controlling the basic ventilation rate to be 0.001-0.01 vvm, and respectively feeding a CellTurbo Feed2 fed-batch culture medium according to the proportion (V / V) of 2-5% on the fourth, sixth, eighth and tenth days after inoculation.
Owner:长春生物制品研究所有限责任公司
Ovulation detection test strip and detection method
PendingCN112730854AHigh detection sensitivityStrong specificityBiological testingAntiendomysial antibodiesCotinine
The invention discloses an ovulation detection test strip. The ovulation detection test strip comprises a PVC bottom plate, a sample pad, a gold label pad, a nitrocellulose film and a water absorption pad, wherein the nitrocellulose film is provided with a detection line T1, a detection line T2 and a quality control line, the detection line T1 is coated with a mouse anti-human beta LH monoclonal antibody 1A4, the detection line T2 is coated with a cotinine antigen, and the quality control line is coated with a goat anti-chicken IgY polyclonal antibody; and a colloidal gold mouse anti-human beta LH monoclonal antibody 6B8 conjugate, a colloidal gold chicken IgY antibody conjugate and a colloidal gold cotinine monoclonal antibody conjugate are cured on the gold label pad. The test strip is high in detection sensitivity and good in specificity, abnormal interpretation results caused by crossing of LH with follicle stimulating hormone (FSH) and thyroid stimulating hormone (TSH) can be avoided, detection results are more accurate, and monitoring of the smoking metabolite cotinine in urine is combined. The invention further discloses an ovulation detection method, the steps are simple, and the test result is accurate.
Owner:杭州新脉生物科技有限公司
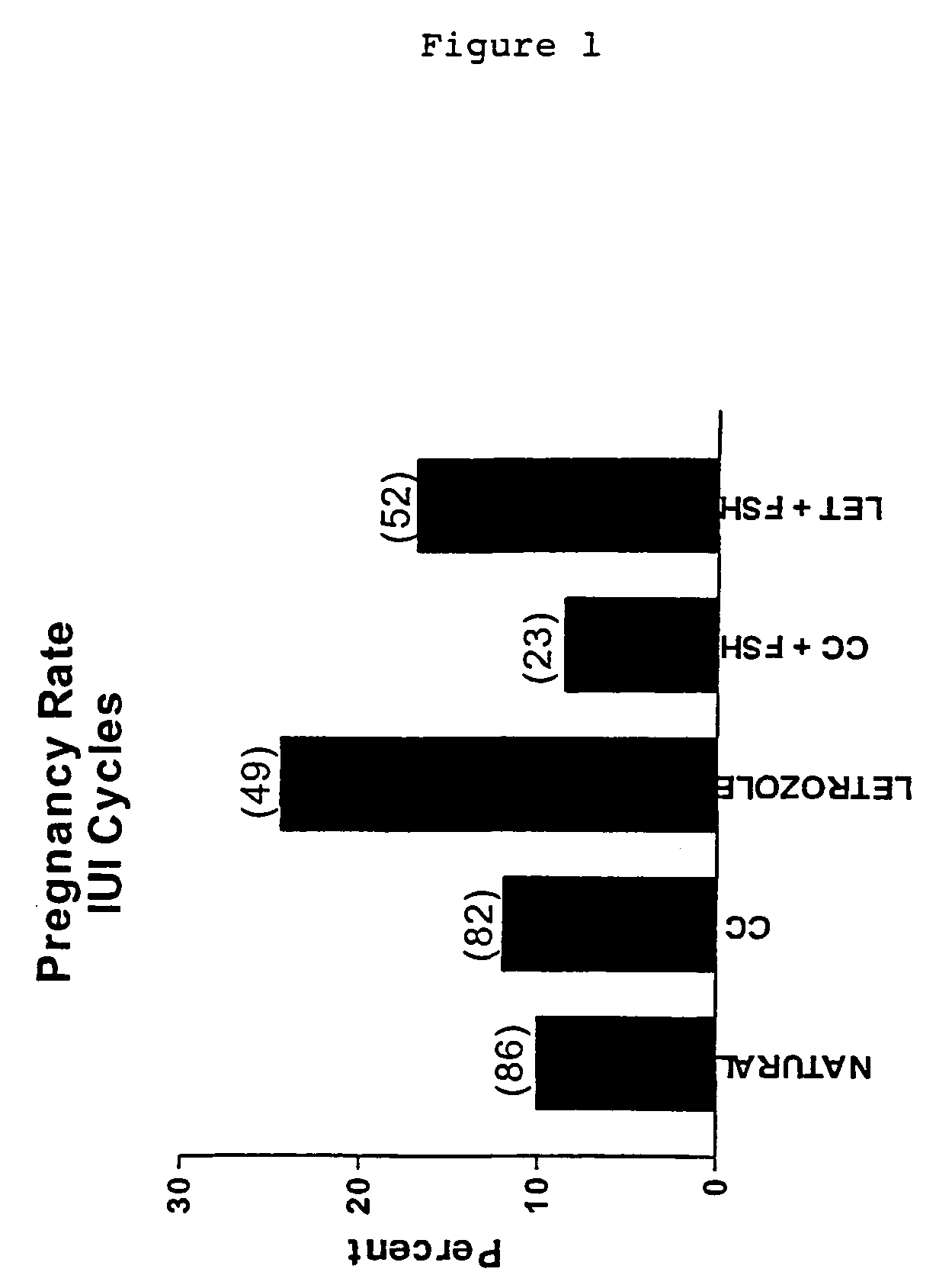
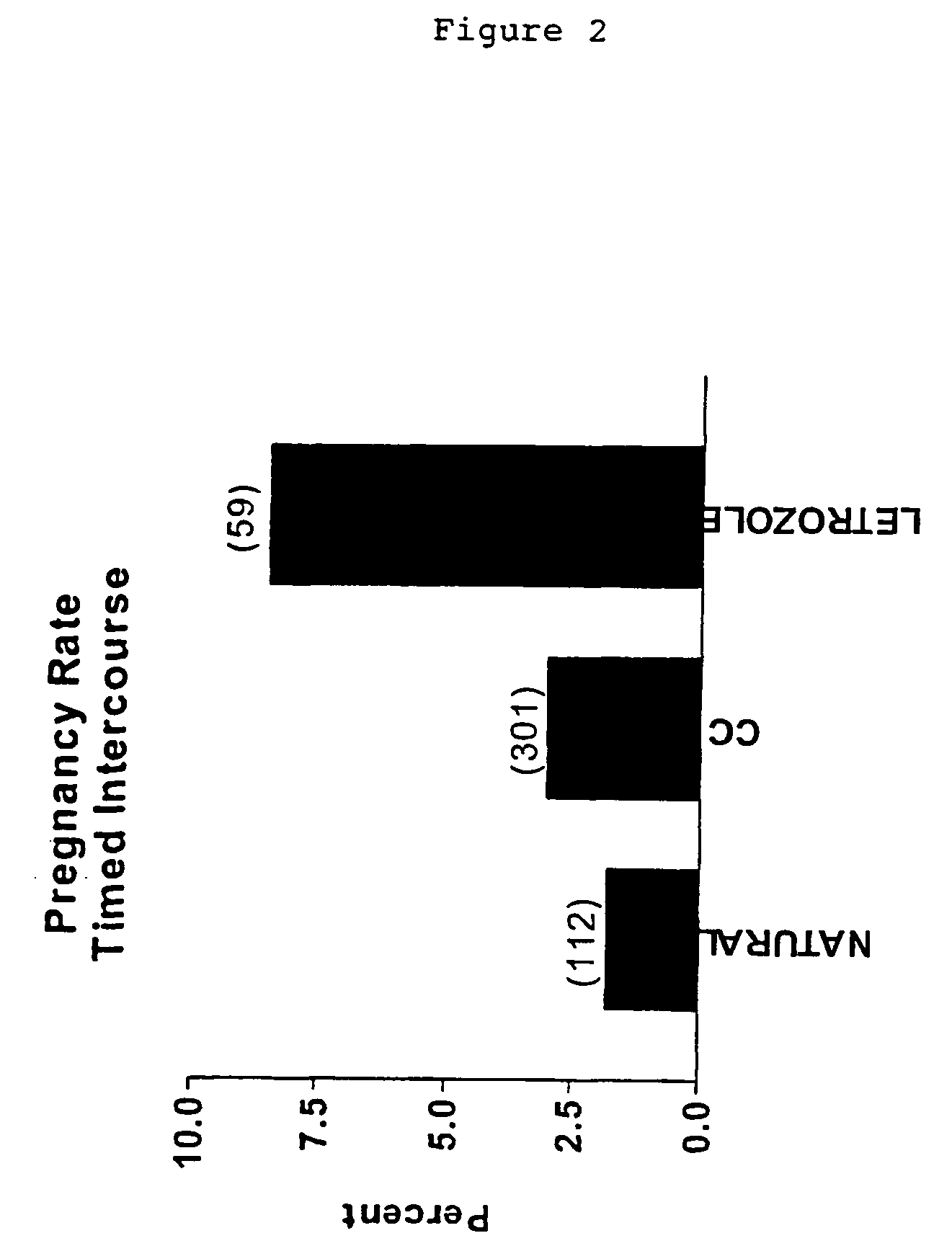
Multiple dose aromatase inhibitor for treating infertility
InactiveUS7820706B2Increasing gonadotropin secretionEliminate side effectsBiocidePeptide/protein ingredientsPhysiologyMenstrual cycle
A method of inducing ovulation in a female suffering from anovulatory infertility which comprises administering to said female two or more daily doses of at least one aromatase inhibitor. A method for augmenting ovulation in an ovulating female suffering from unexplained infertility or another type of ovulatory infertility which comprises administering to said female two or more daily doses of at least one aromatase inhibitor early in one or more menstrual cycles. A method of substantially reducing dosage levels of follicle stimulating hormone (FSH) for administration to a female undergoing infertility treatment which comprises administering a combination of two or more daily doses of at least one aromatase inhibitor (AI) with a plurality of daily doses of follicle stimulating hormone (FSH). A method of increasing response to a follicle stimulating hormone from a female who is a poor responder to follicle stimulation, which comprises administering a combination of two or more daily doses or at least one aromatase inhibitor (AI) with a plurality of daily doses of follicle stimulating hormone (FSH). Also disclosed are related pharmaceutical preparations and uses.
Owner:ARES TRADING SA
Recombinant CHO cell strain with high expression of human follicle stimulating hormone and construction method thereof
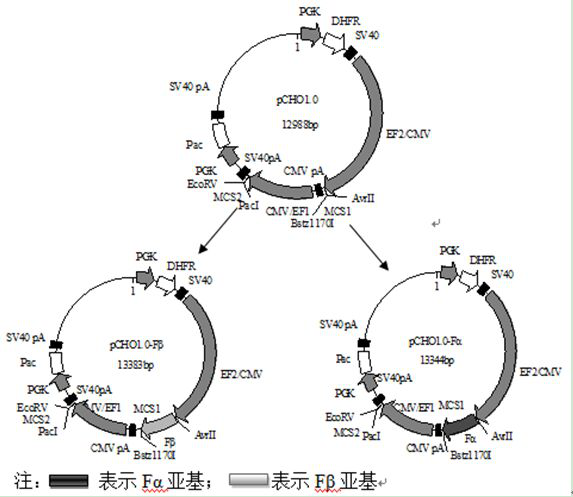
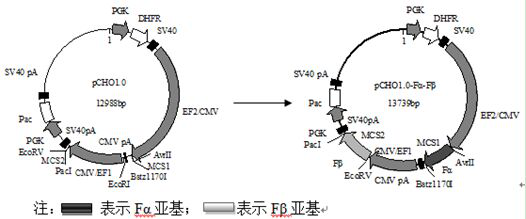
PendingCN113512537AHigh expressionQuality improvementGenetically modified cellsDepsipeptidesProtein targetPregnancy
The invention relates to the technical field of auxiliary devices of a pregnancy assisting technology, in particular to a recombinant CHO cell strain with high expression of human follicle stimulating hormone and a construction method thereof. The recombinant CHO cell strain is high in purity, low in cost and stable in biological activity, and comprises a CHO-S cell strain, a pCHO1.0 vector, a high expression element and a target protein amino acid sequence.
Owner:长春生物制品研究所有限责任公司
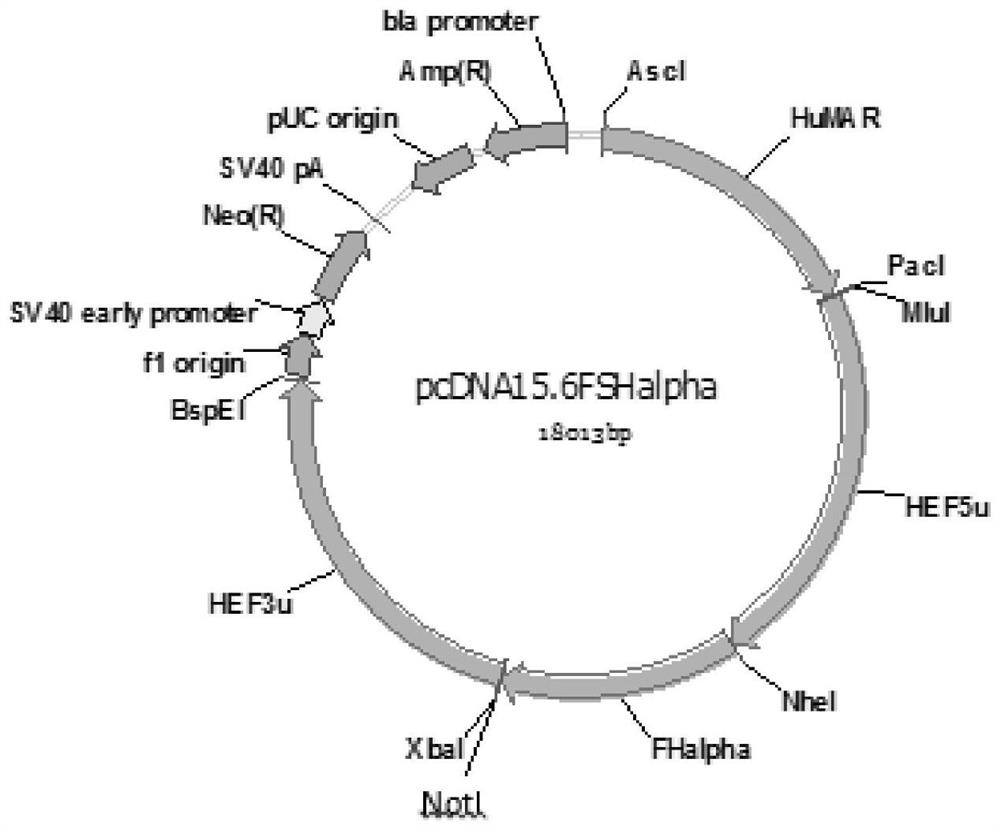
Recombinant human follicle stimulating hormone and preparation method thereof
PendingCN112195194AEfficient captureEfficient removalPeptide preparation methodsNucleic acid vectorRecombinant human follicle stimulating hormoneCricetulus
The invention discloses a recombinant human follicle stimulating hormone and a preparation method thereof. The preparation method comprises the following steps: jointly transfecting a constructed expression vector containing an FSHalpha subunit and an expression vector containing an FSHbeta subunit into Chinese hamster ovary cells, screening out stably expressed cell strains, and purifying and culturing the cell strains to obtain a culture solution, thereby obtaining the recombinant human follicle stimulating hormone, wherein the C-terminus of the FSHbeta subunit is connected with CTP. According to the preparation method of the recombinant human follicle stimulating hormone, hFSHalpha and hFSHbeta sequences comprising introns are cloned from human embryonic kidney 293 cell genomes respectively, CTP sequences are connected to the terminus of the beta subunit in a primer extension mode to obtain hFSHbeta-CTP, then the two subunit sequences are transfected to CHO-S cells, rhFSH-CTP is obtained through expression, the half-life period of the rhFSH-CTP obtained by expression in vivo is longer, the final half-life period is 2-3 times that of recombinant FSH, and the biological activity is higher.
Owner:北京双鹭生物技术有限公司 +2
Use of Euycoma longifolia extract in alleviating symptoms and/or conditions associated with hormonal imbalance in females
ActiveUS11058737B2Safe and effective and of symptomSafe and effective treatmentEndocrine system disorderPlant ingredientsHormonal imbalanceLutenizing hormone
The present invention is directed to a new kind of medicinal value or health care function of Eurycoma longifolia extracts, particularly in the treatment or alleviation symptoms and / or conditions associated to hormonal imbalance in females, including menopause and its related symptoms. In one aspect, the present invention discloses the use of a composition comprising a therapeutically effective amount of Eurycoma longifolia extract in the manufacture of a medicament for the alleviation of symptoms and / or conditions associated to hormonal imbalance in females, including menopause and its related symptoms. The alleviation of the symptoms and / or conditions according to the present invention is characterised by inducing a change in the hormonal contents of estrogen, progesterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH). Also disclosed is the use of a pharmaceutical composition comprising the therapeutically effective amount of Eurycoma longifolia extract.
Owner:BIOTROPICS MALAYSIA BERHAD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com