Recombinant human follicle stimulating hormone and preparation method thereof
A human follicle-stimulating hormone and ovarian cell technology, applied in the field of bioengineering, can solve the problems of low biological activity and poor purification effect of recombinant human follicle-stimulating hormone, and achieve the effects of stable protein quality, increased sialic acid content and improved safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
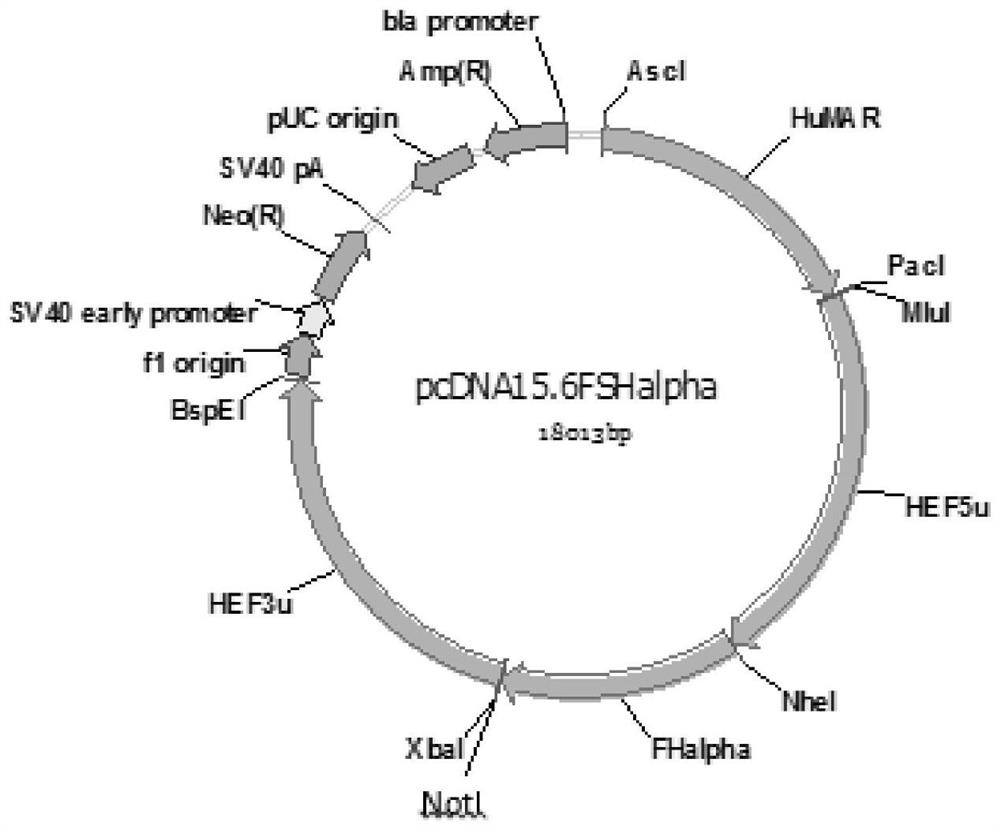
[0043] Embodiment 1, construction of rhFSHα subunit gene expression vector
[0044] The gene sequence of the coding region of the FSHα subunit of the present invention is shown in SEQ ID NO: 1, and the amino acid sequence of the encoded protein is shown in SEQ ID NO: 10. The amplification primers of the FSHα subunit gene of the present invention are to add NheI and NotI enzyme cutting sites on the primers at both ends of the gene of the FSH-α subunit, and the designed primers are shown in Table 1.
[0045] Table 1
[0046]
[0047] The above primers I and II were used to amplify the rhFSHα subunit gene using human 293 cell genomic DNA as a template, and Pfu DNA polymerase was used for PCR amplification.
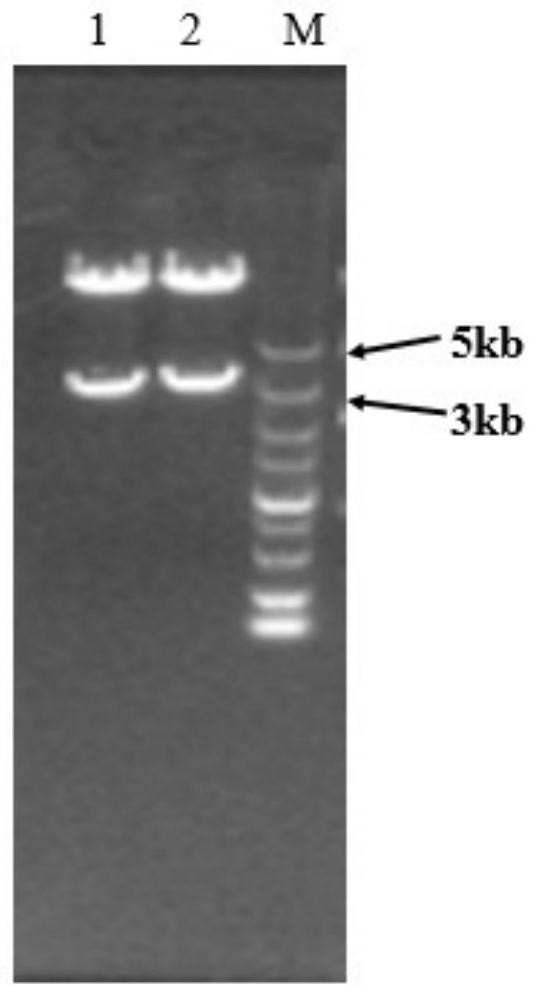
[0048] The PCR amplification conditions are: 95°C for 5min, 94°C for 30s, 60°C for 30s, 72°C for 2min, a total of 30 cycles of amplification, and 72°C for 5min to amplify the DNA fragment of the FSHα subunit gene. Recover on 1% agarose gel, then digest the DNA fragment o...
Embodiment 2
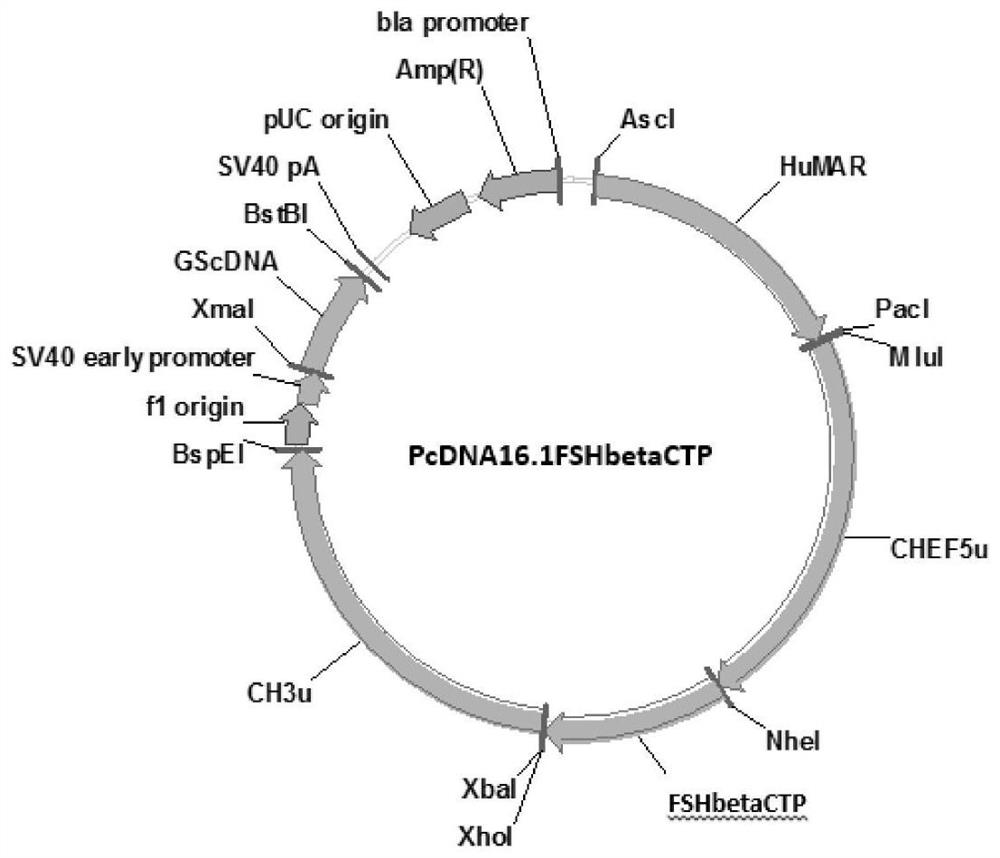
[0050] Embodiment 2, construction of rhFSHβ-CTP subunit gene expression vector
[0051] The C-terminus of the FSHβ subunit of the present invention is linked with the gene sequence of the coding region of CTP as shown in SEQ ID NO: 2, and the amino acid sequence of the encoded protein is shown in SEQ ID NO: 11. The primers at both ends of the rhFSH β-CTP subunit gene of the present invention are added with Nhe I and Xho I restriction sites, and the primer sequences are designed with the rhFSH β-CTP subunit gene. The primer sequences from primer I to primer V are shown in Table 2 .
[0052] Table 2
[0053]
[0054] Using the DNA fragment of the FSHβ gene as a template, primer I (SEQ ID NO: 3) and primer II (SEQ ID NO: 4) were used to amplify, and the DNA polymerase used pfuDNA polymerase from TIANGEN Company.
[0055] PCR amplification conditions are: 95°C for 4min, 94°C for 30s, 60°C for 30s, 72°C for 100s, amplifying for 30 cycles, and extending at 72°C for 5min to obta...
Embodiment 3
[0060] Example 3, Cell Transfection and Screening of Highly Expressed Cell Line rhFSH-CTP
[0061] Transform the recombinant expression vectors pcDNA15.6-FSHα and pcDNA16.1-FSHβ-CTP into Escherichia coli DH5α competent cells, rejuvenate the transformed bacteria with LB liquid selection medium containing ampicillin and expand to 100mL for mass culture, shaking at 37°C After culturing overnight, extract and purify the plasmid, adjust the concentration of the purified plasmid DNA to 1 μg / μL, and mix the recombinant expression plasmids pcDNA15.6-FSHα and pcDNA16.1-FSHβ-CTP at a molar ratio of 1:1.
[0062] One day before transfection, the host cell CHO-S cells were divided into 0.5×10 6 The seeding density of cells / mL was subcultured to 20 mL of fresh medium. On the day of electroporation, three groups of cells in the logarithmic growth phase were collected in parallel, and the number of cells in each group was 1×10 7 After removing the medium by centrifugation, wash the cells w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com