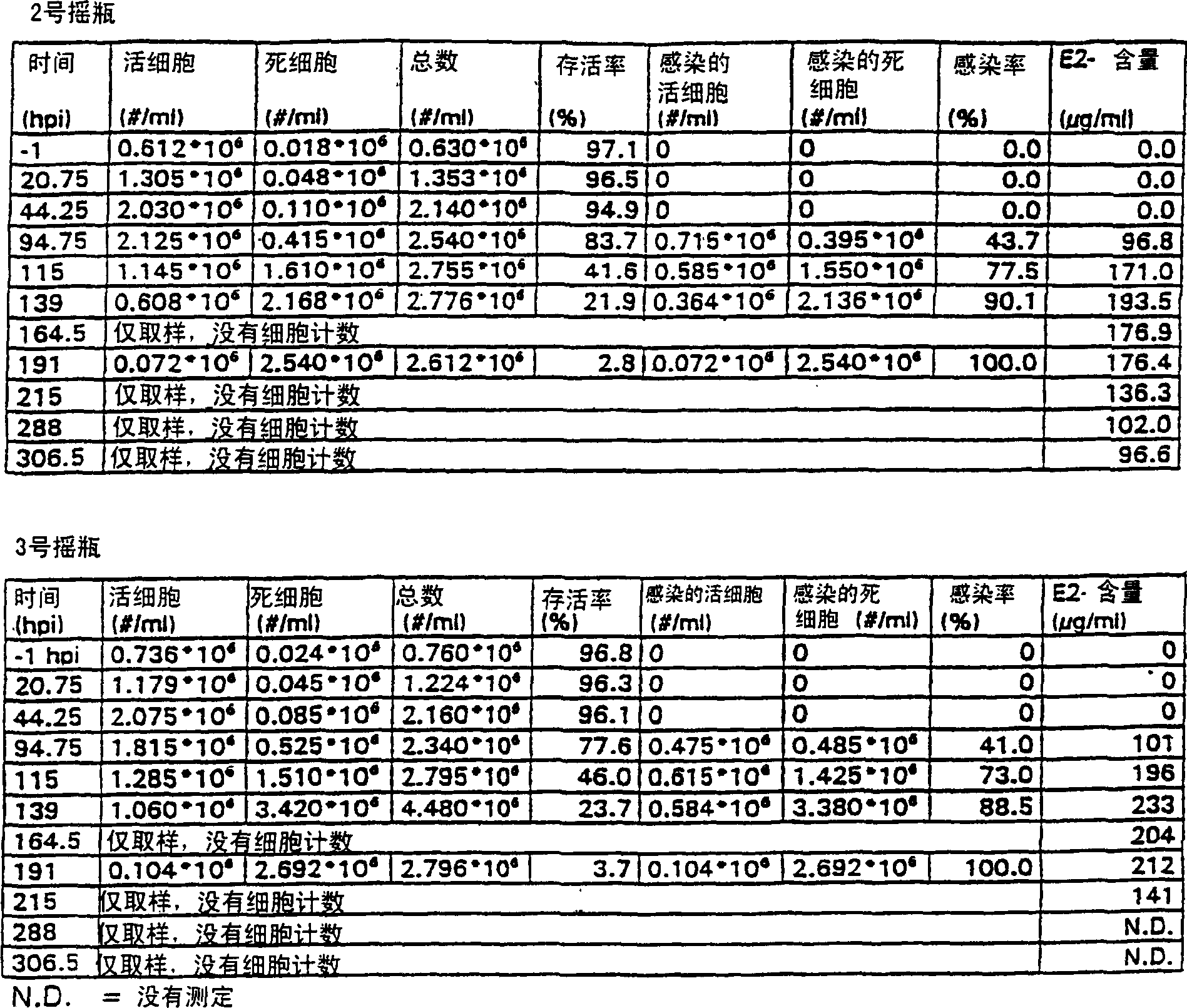
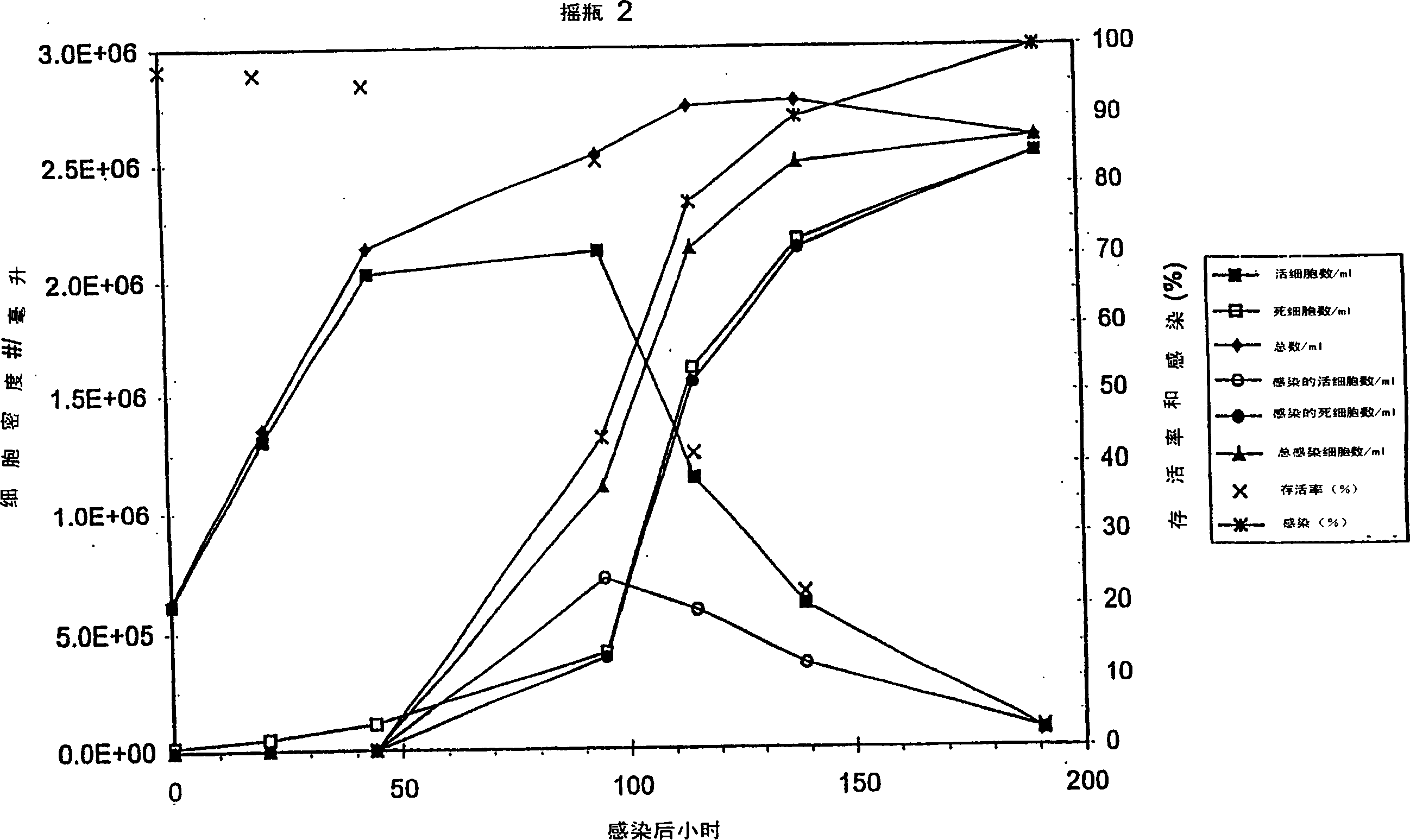
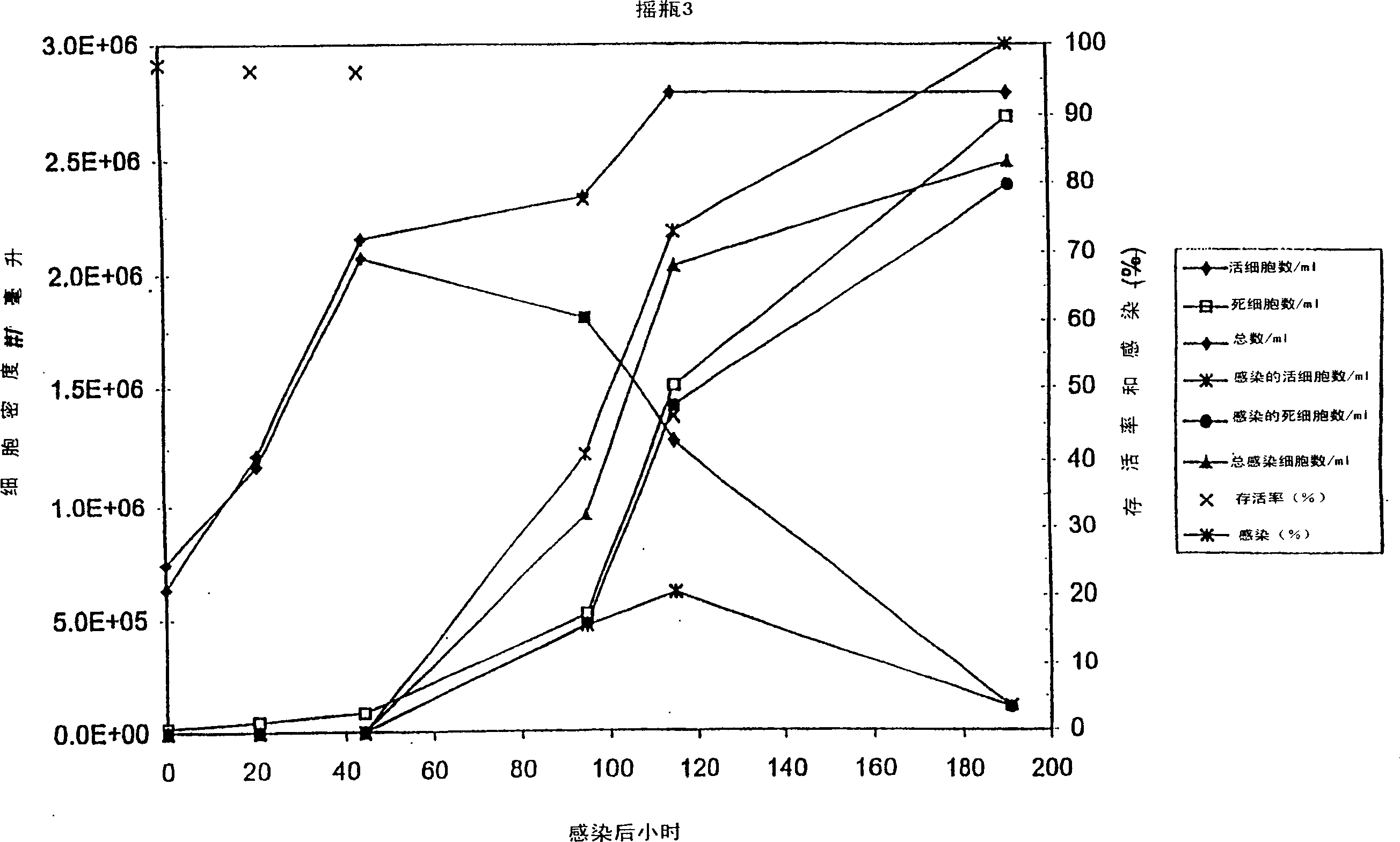
Protein expression in baculovirus vector expression system
A baculovirus, recombinant baculovirus technology, applied in the field of enhancing protein or polypeptide expression, can solve the problem of not considering DNA and RNA accumulation and infection cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] specific implementation plan
[0036] Pestiviruses of the Flaviviridae family generally include classical swine fever virus (CSFV), border disease virus (BDV), and bovine viral diarrhea virus (BVDV). The genomes of several BVDV and CSFV strains have been sequenced (Renard et al., 1987, European Patent Application 0208672; Collett et al., 1988, Virology 165, 191-199; Deng and Brock, 1992, Virology 1991, 865- 679; Meyers et al., 1989, Virology 171, 555-567; Moormann et al., 1990, Virology 177, 184-188). For BDV, only incomplete genome nucleotide sequences were available. The pestivirus genome is a positive-strand RNA molecule of about 12.5 kb containing a large open reading frame. The open reading frame translates into a hypothetical polyprotein of approximately 4,000 amino acids, which is processed by viral and cellular-encoded proteases. The open reading frame is flanked by two small, highly conserved untranslated regions that may be involved in genome replication. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com