Stable pemetrexed arginine salt and compositions comprising it
a technology of pemetrexed arginine salt and pemetrexed salt, which is applied in the direction of biocide, drug composition, organic chemistry, etc., can solve the problems of unstable final product stability, problems such as problems, and formulating water soluble pemetrexed salts has not proved to be an easy task
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0047]To 500 mg of pemetrexed diacid and 407 mg of arginine, 6 ml of water was added. The mixture was stirred until a clear solution was formed. This took approximately 5 minutes. The solvent was evaporated and the residue was dried over night at 40° C. under vacuum.
[0048]XRPD test confirmed amorphous character of the product.
[0049]TGA curve exhibits gradual mass loss of 4.738% of water between 25 and 220° C.
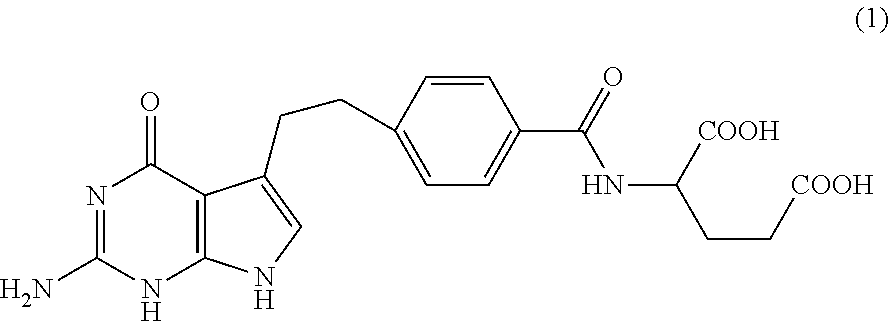
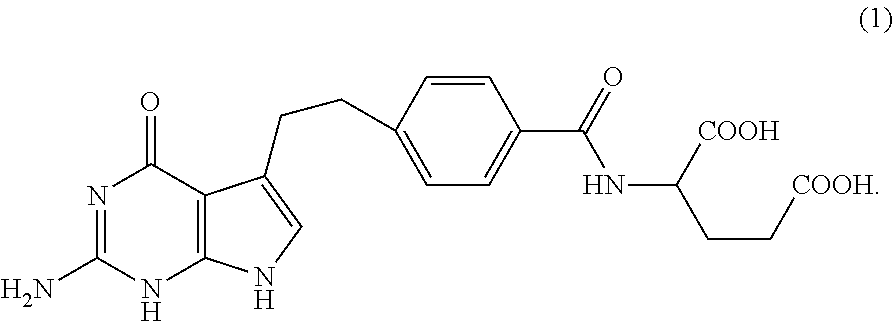
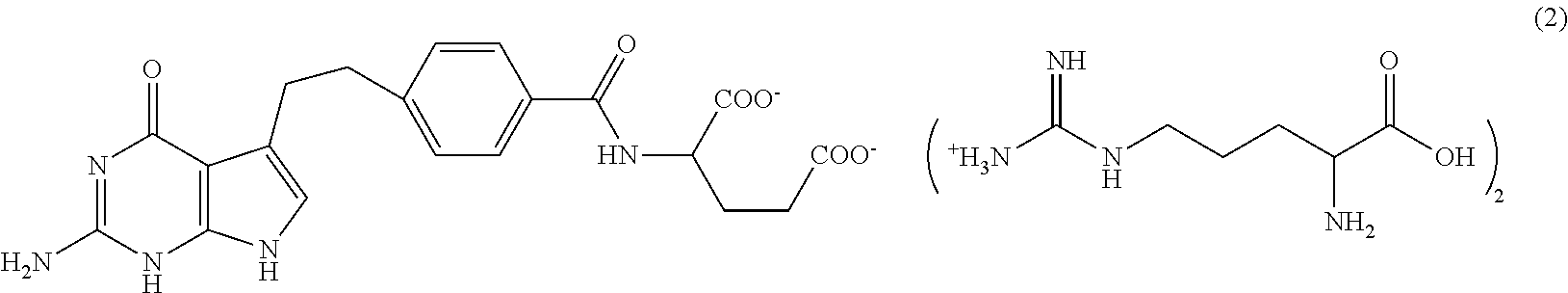
[0050]NMR confirmed the structure of bis-arginine salt (formula (2))
example 2
[0051]1000 mg of pemetrexed (diacid) and 815 mg of arginine was added to 6 ml of water. The mixture was stirred at ambient temperature until a clear solution was formed.
[0052]The solution was divided into 6 fractions and the following cosolvents were added to the respective fractions:
[0053].01 3 ml of methanol
[0054].02 3 ml of ethanol
[0055].03 2 ml of isopropanol
[0056].04 2 ml of acetonitrile
[0057].05 2 ml of tetrahydrofuran
[0058].06 2 ml of acetone
[0059]The solutions were evaporated on a rotary vacuum evaporator. The solid residues were dried at 40° C. under vacuum over night.
[0060]Analysis of the material by XRPD has proven an amorphous material.
example 3
[0061]Process for making unit dose lyophilized composition (50 mg pemetrexed per vial):
[0062]Under inert atmosphere of nitrogen, 875.0 mg of pemetrexed (diacid) was dissolved in a solution of 731.0 mg arginine (2.1 molar equivalents related to pemetrexed) in 30 ml of Water for injections under stirring. After that, 875 mg of mannitol was added and dissolved. The pH was adjusted to about 7.5 by 1 M hydrochloric acid and the weight of the solution was adjusted to 35 gram amount by Water for injections. The solution was filtered through PVDF filter 0.2 microns, filled in clear 10 R vials per 2 grams and freeze dried according to the program presented in the table.
DurationTemperatureVacuumSafety PressureProcess Phase(hh:mm)(° C.)(mbar)(mbar)Loading00:00+5Fast freezing01:00−45Freezing01:30−45Freezing00:45−15Annealing01:00−15Freezing01:30−45Freezing02:00−45Evacuation 100:10−450.370.63Sublimation05:00+50.370.63Sublimation06:15+50.370.63Evacuation 200:10+50.060.63Second drying05:35+300.060....
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com