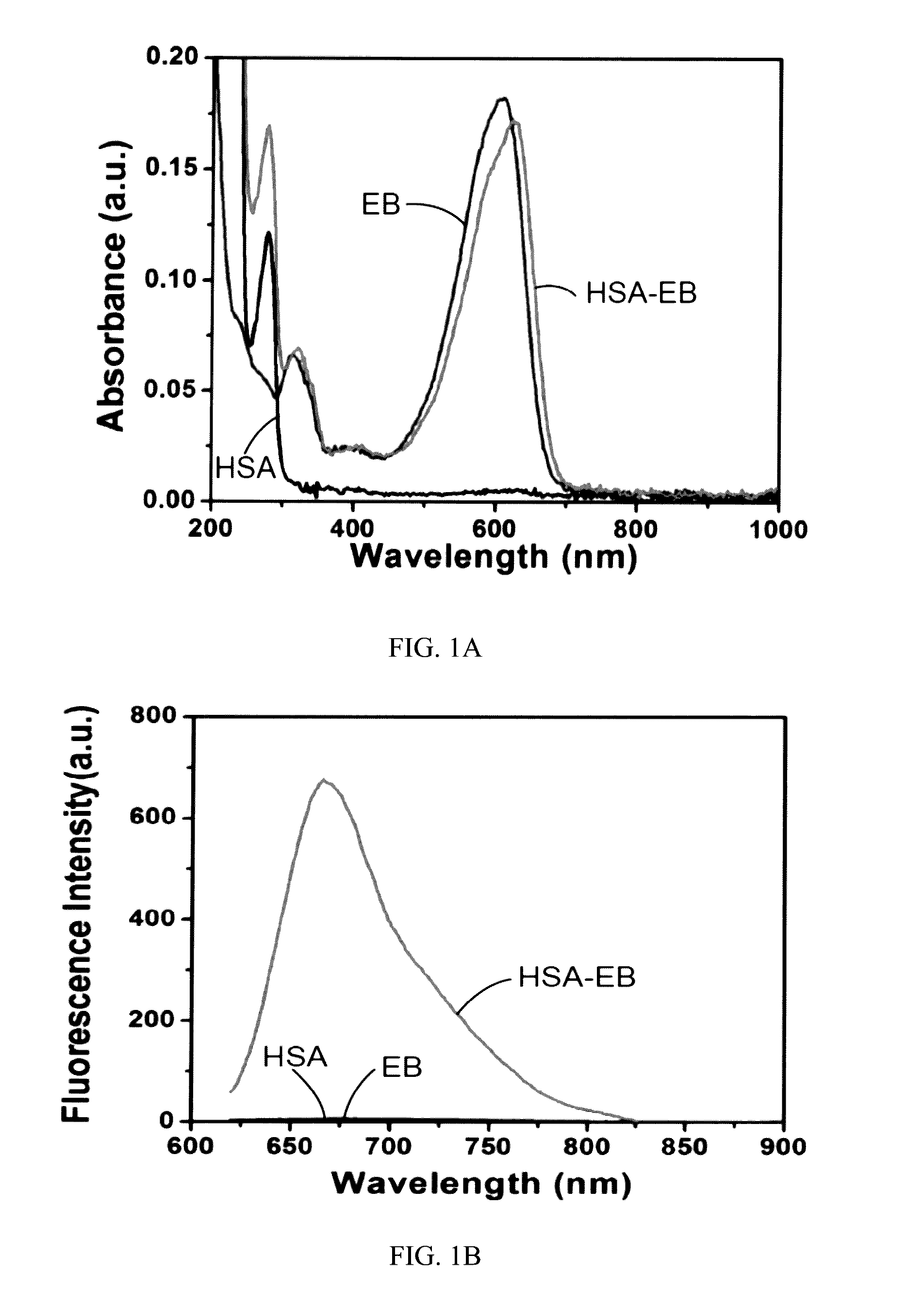
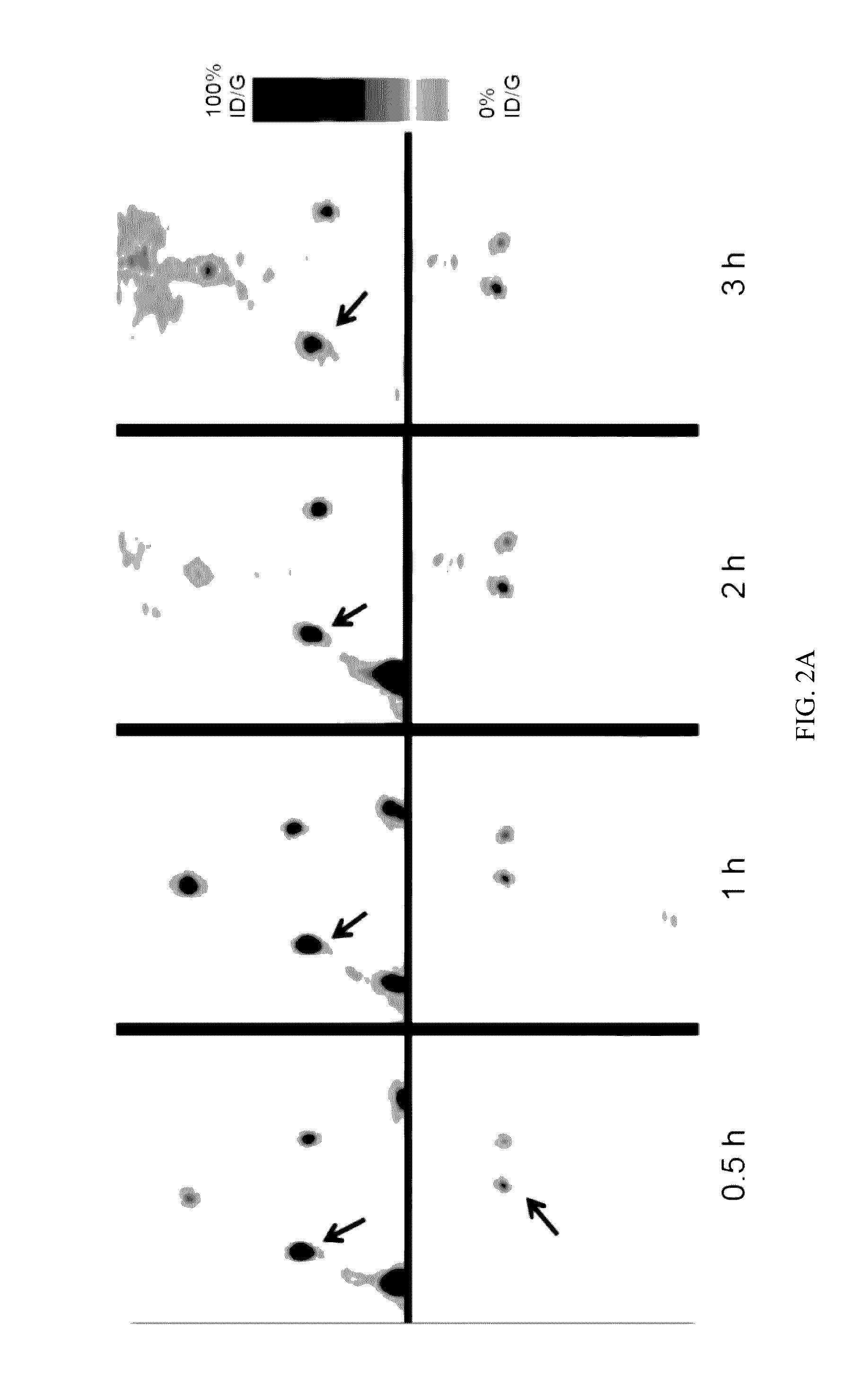
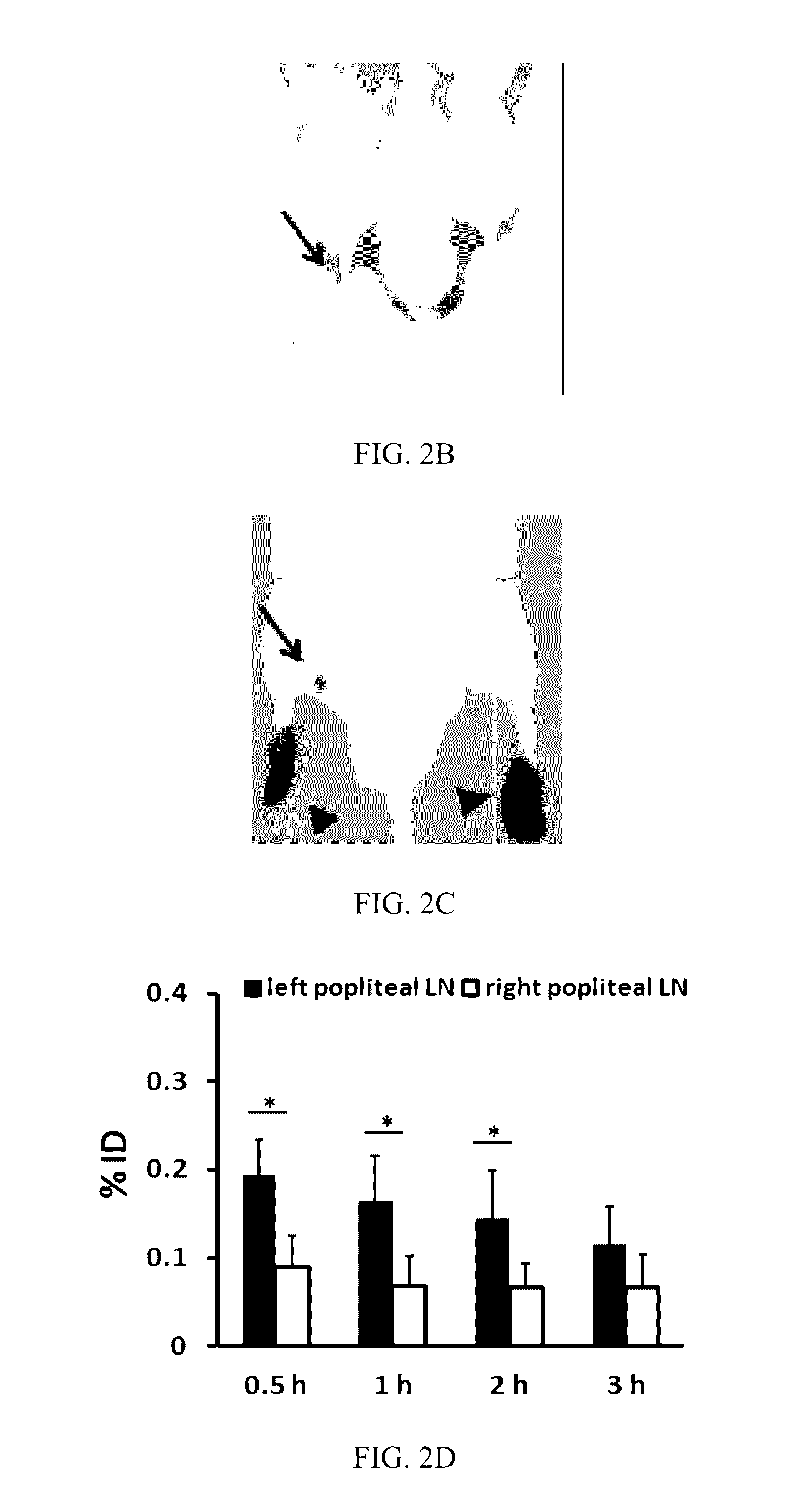
Labeled evans blue dye derivative for in vivo serum albumin labeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0162]This example demonstrates a synthesis of the compound:
in accordance with an embodiment of the invention.
[0163]To a 4 mL glass vial containing 20.0 mg of o-tolidine (94 μmol) and 20.0 mg of 1, 4, 7-triazacyclononane-N,N′,N″-triacetic acid-3HCl (“NOTA”) (48 mop in 1 mL of DMSO was added 3.6 μL of diethyl cyanophosphonate (24 μmol) and 25 μL of diisopropylethylamine (DIPEA). The mixture was stirred at room temperature for 40 min and another 3.6 μL of diethyl cyanophosphonate was added and stirred at room temperature overnight. The mixture was then purified with semi-preparative HPLC. The peak containing the desired product was collected (Rt=10.0 min) and the solution was frozen over dry ice and lyophilized overnight to give 12.2 mg pure product in 26.4% yield. LC-MS (C26H35N5O5): [MH]+=498.2467 (m / z), calc: 497.2638.
example 2
[0164]This example demonstrates a synthesis of the compound:
in accordance with an embodiment of the invention.
[0165]To a 20 mL glass vial containing 2.5 mg of NOTA-tolidine (5.0 μmol) in 0.3 mL of water was added 18 μmol of HCl in 0.1 mL of water. The mixture was cooled in ice bath and 0.5 mg of sodium nitrite (7.2 μmol) in 0.1 mL of water was added to the vial. The mixture was stirred in ice bath for 20 min and the yellow diazonium salt solution was added dropwise to another vial in ice bath containing 4.0 mg of 1-amino-8-naphthol-2,4-disulfonic acid (10.0 μmol) and 2.4 mg of sodium bicarbonate (28.5 μmol) in 0.2 mL of water. The mixture was stirred in ice bath for 2 h and purified with semi-preparative HPLC. The product (denoted as NEB) was collected (Rt=19.0 min) and lyophilized overnight to give 1.4 mg pure product in 46.6% yield. LC-MS (C36H41N7O12S2): [M-H]−=826.2415 (m / z), calc: 827.2255.
example 3
[0166]This example demonstrates a synthesis of the compound:
in accordance with an embodiment of the invention.
[0167]To a 1 mL plastic tube containing 3 μL of 2 mM aluminum chloride in 0.5 M pH 4.0 sodium acetate buffer and 6 μL of 3 mM NEB in 0.5 M pH 4.0 sodium acetate buffer was added 0.13 mL acetonitrile and 0.05 mL of aqueous 18F-fluoride (0.3-0.9 GBq). The mixture was vortexed and heated in a 105° C. heating block for 10 min. The vial was cooled, and the solution was diluted with 10 mL of water and trapped on a Varian Bond Elut C18 column (100 mg). The radioactivity trapped on the C18 column was eluted with 0.3 mL of 80% ethanol / water containing 1 mM HCl. The ethanol solution was evaporated with argon flow, and the final product was dissolved in PBS and analyzed by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com