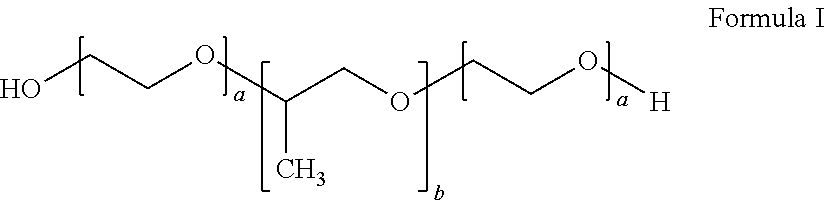
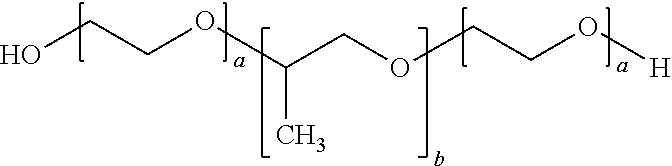
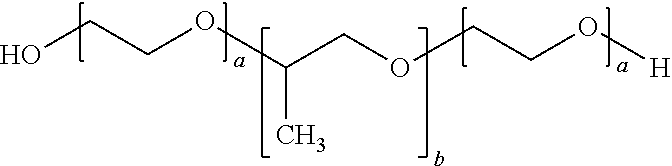
Topical antiseptic system
a topical antiseptic and system technology, applied in the field of topical antiseptic systems, can solve the problems of increasing material and procedural costs as well as time for health care professionals, and general unsuitability for a variety of applications including the treatment of mucosal tissue or wounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
Preparation of Topical Antimicrobial Composition
[0277]Step 1
[0278]In an appropriate size of beaker, combine jojoba esters (40 parts), cetyl alcohol (2 parts) and a mixture of 65% glyceryl stearate / 35% PEG-100 stearate (80 parts). Heat the mixture to 72±3° C. until all ingredients are melted and a clear solution is obtained. Maintain the temperature at 72±3° C.
[0279]Step 2
[0280]In a manufacturing vessel, add purified water (qsad 1000 parts) and EDTA, disodium salt (2 parts). Heat the mixture to 72±3° C. with propeller mixing. Continue mixing until the EDTA disodium salt is completely dissolved. Maintain the temperature at 72±3° C.
[0281]Step 3
[0282]With mixing, add laureth −4 (40 parts), benzalkonium chloride (as a 50% aqueous solution) (2 parts) and PVP / Iodine (11 parts) to the manufacturing vessel. Continue mixing until the PVP-Iodine is dissolved and a homogeneous solution is obtained. Maintain the temperature at 72±3° C.
[0283]Step 4
[0284]Add the solution from Step 1 to the manufac...
example b
Procedure
[0289]Water (84.4 g) was placed in a sanitized mixing vessel. EDTA disodium salt (0.1 g) was added and dissolved into the water with continued mixing. Hydroxyelthylcellulose (HEC, 0.25 g) was slowly added with stirring to avoid clumping. NaOH (0.2 g of 20% by weight aqueous solution) was added and mixed until batch was completely smooth and free of “fish eyes”. The remaining ingredients (Floraesters K=20W jojoba (2.6 g), Laureth-4 (2 g), benzalkonium chloride (0.01 g), and sodium iodide (0.5 g) were independently added to ensure that each ingredient was dissolved before the addition of the next. PVP-iodine (9.726 g) was very slowly added to the mixture with mixing carried out as to avoid entrapment of air. PVP-I % was calculated according to “Available Iodine AS IS” assay found on COA (11.31%) for a total of 1.1% Iodine. The batch was mixed until batch was completely homogeneous.
APPEARANCE: Semi-viscous clear liquid
COLOR: Dark reddish / brown
Initial...
example c
[0292]Water (74.5 g) was placed in a sanitized mixing vessel. EDTA disodium salt (0.1 g) was added and dissolved into the water with continued mixing. Pluracare F 127 (analogous material to Poloxamer 407, 10 g) was slowly added to avoid introduction of excess air. PVP-iodine (9.73 g) was very slowly added to the mixture with mixing carried out as to avoid entrapment of air. PVP-I % was calculated according to “Available Iodine AS IS” assay found on COA (11.31%) for a total of 1.1% Iodine. NaOH (0.6 g of 20% by weight aqueous solution) was added and mixed until batch was completely smooth and free of “fish eyes”. The remaining ingredients (Floraesters K=20W jojoba (2.6 g), Laureth-4 (2 g), benzalkonium chloride (0.01 g), and sodium iodide (0.5 g) were independently added to ensure that each ingredient was dissolved before the addition of the next. The batch was mixed until batch was completely homogeneous.
APPEARANCE: Semi-viscous clear liquid
COLOR: Dark reddish / brown
ODOR: Characteris...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Brookfield viscosity | aaaaa | aaaaa |
| Brookfield viscosity | aaaaa | aaaaa |
| Brookfield viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com