Nickel-free austenitic stainless steel
a technology of austenitic stainless steel and composition, which is applied in the field ofnickel-free austenitic stainless steel composition, can solve the problems of disadvantageous high concentration of manganese, difficult to shape parts large difficulty in shaping by machining and forging, etc., and achieves sufficient corrosion resistance, sufficient corrosion resistance, and facilitating shape operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
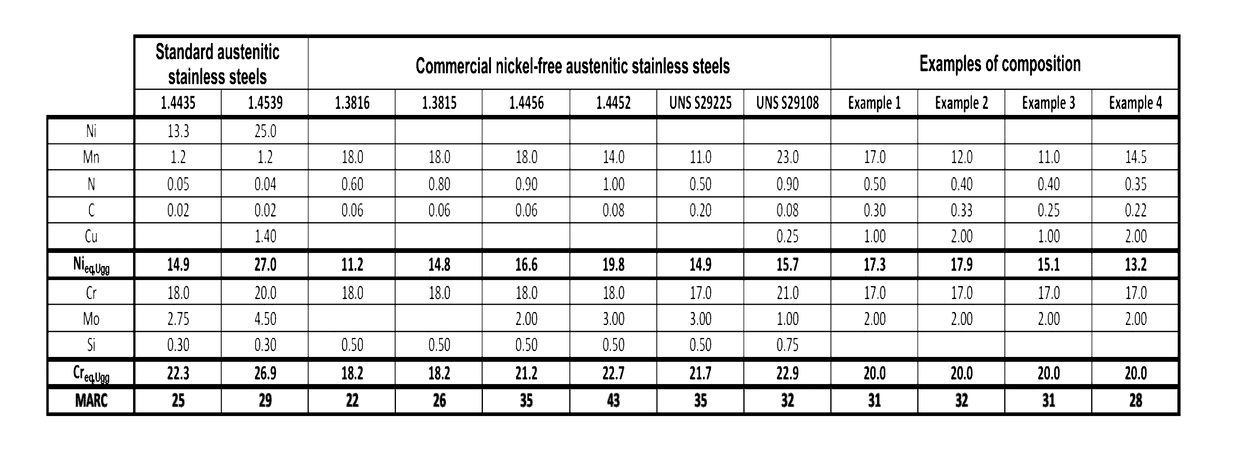
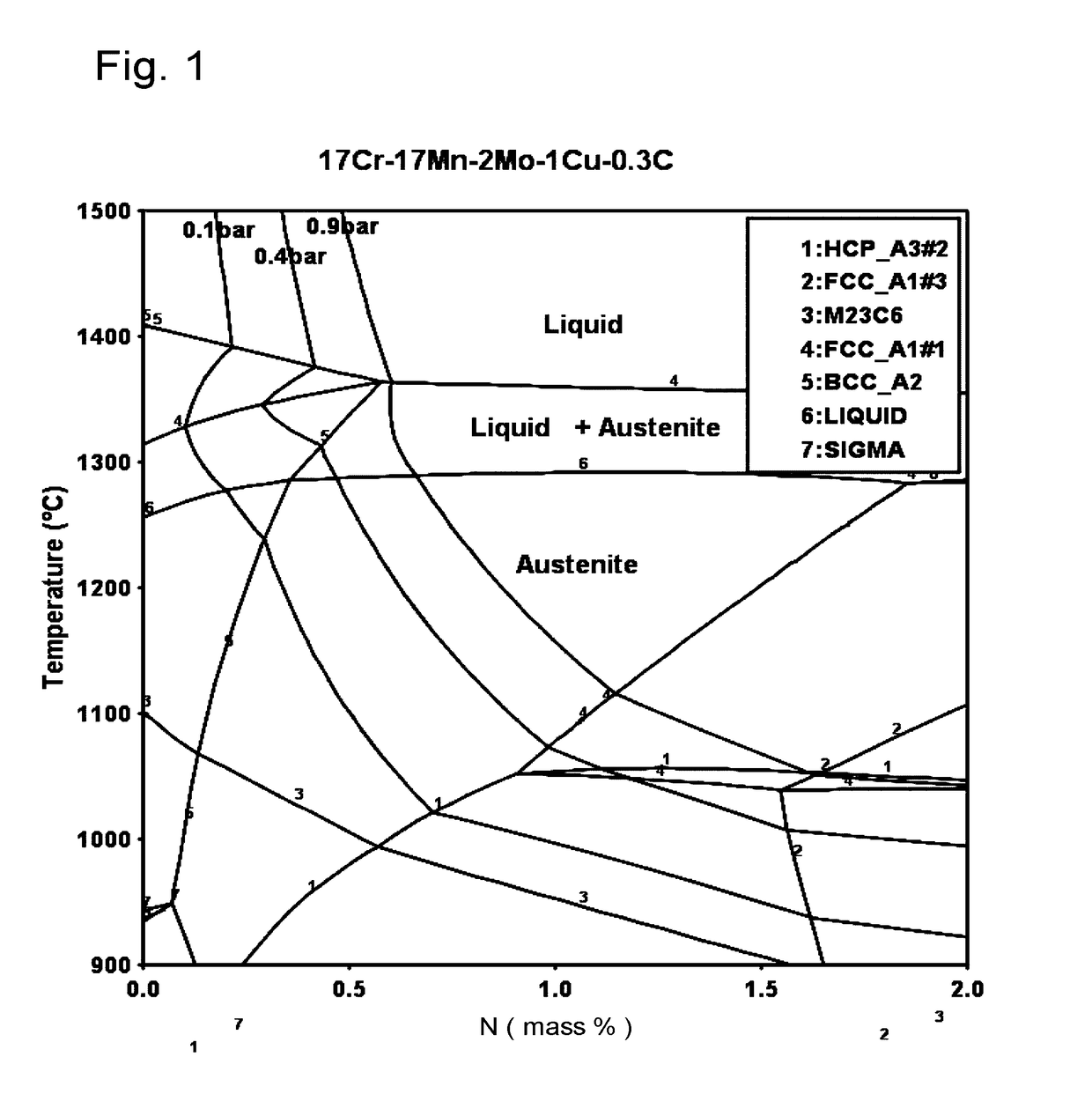
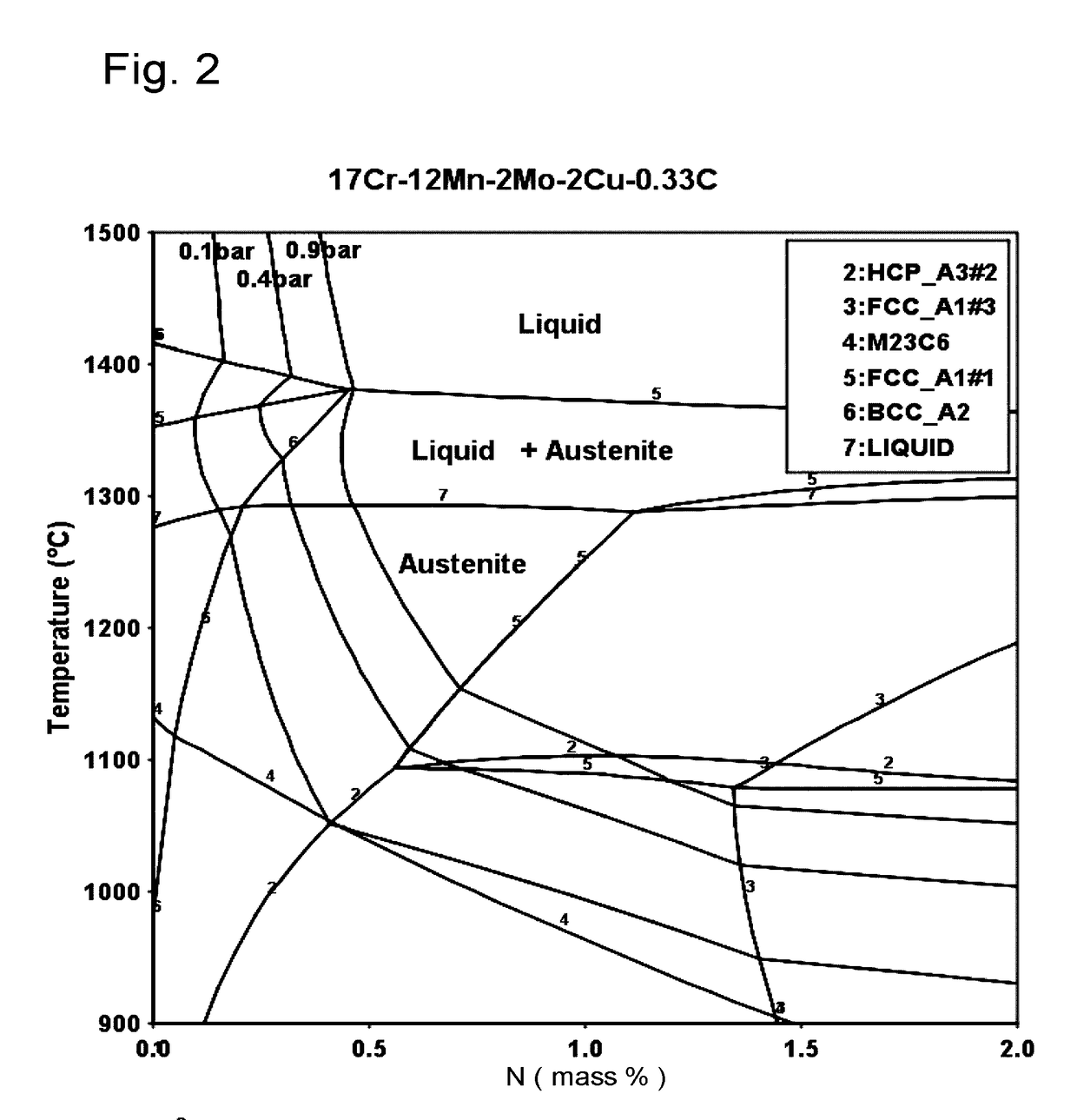
[0074]The present invention proceeds from the general inventive idea which consists in proposing nickel-free austenitic stainless steels representing a very good compromise between machinability and forgeability properties and corrosion resistance, taking account of issues specific to the field of external timepiece parts. Further, the proposed compositions can be obtained by means of conventional metallurgy (foundry), in particular at ambient atmospheric pressure, which is very advantageous from the point of view of production costs, or by powder metallurgy with very high densities after sintering. The concentrations of alphagenous elements, such as chromium and molybdenum, are defined to obtain sufficient corrosion resistance. The concentrations of manganese, carbon and nitrogen are sufficiently low to enhance the machinability and forgeability properties of the resulting alloys, but sufficiently high to obtain the alloy by melting and solidification at atmospheric pressure or to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| austenitic temperature | aaaaa | aaaaa |
| austenitic temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com