Submucosal gastric implant device and method
a gastric implant and submucosal technology, applied in the field of submucosal gastric implant devices and methods, can solve the problems of tissue damage, current gastric electrical stimulation procedures are relatively invasive, etc., and achieve the effects of preventing rotation, promoting encapsulation or tissue ingrowth, and small profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
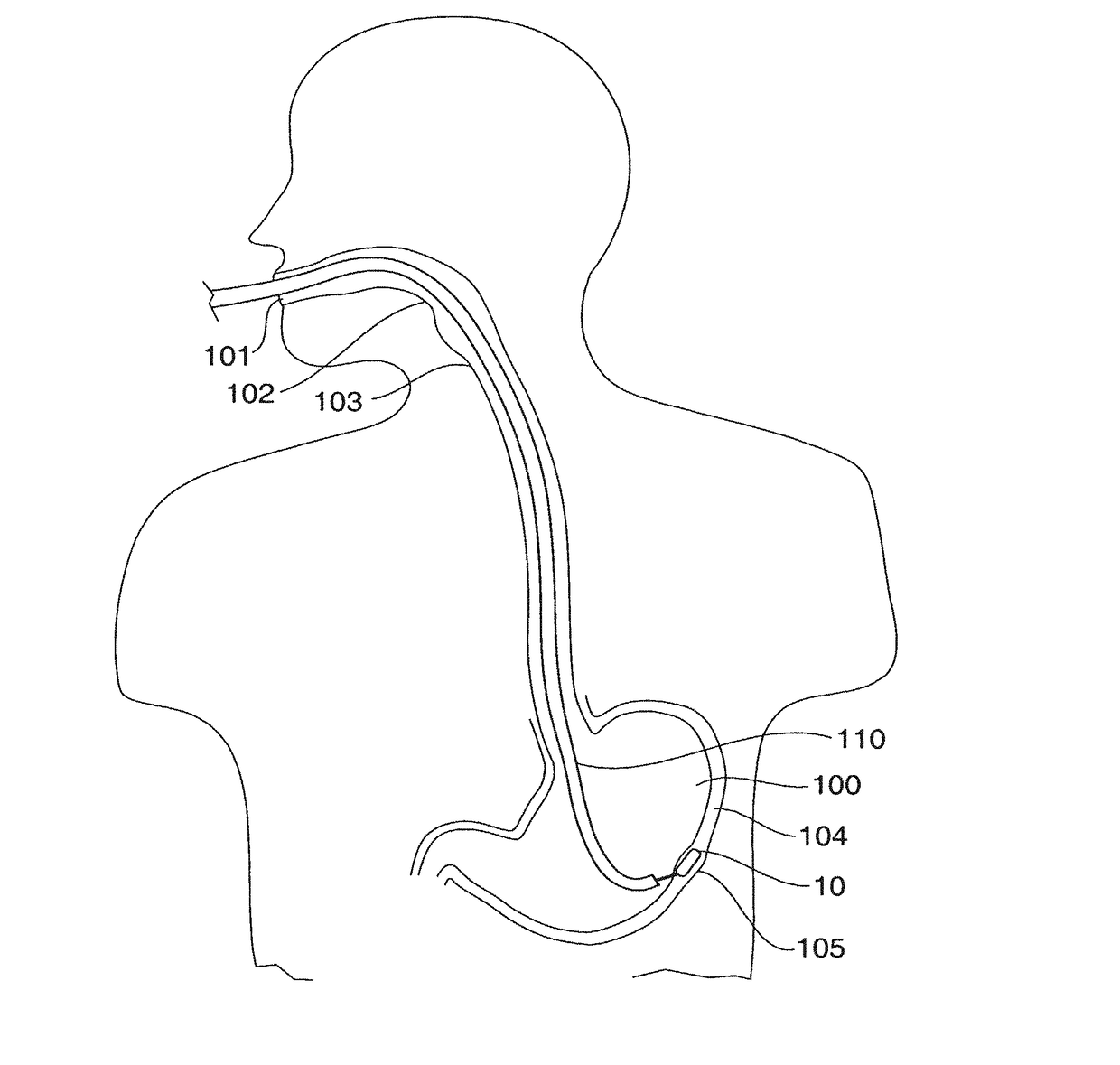
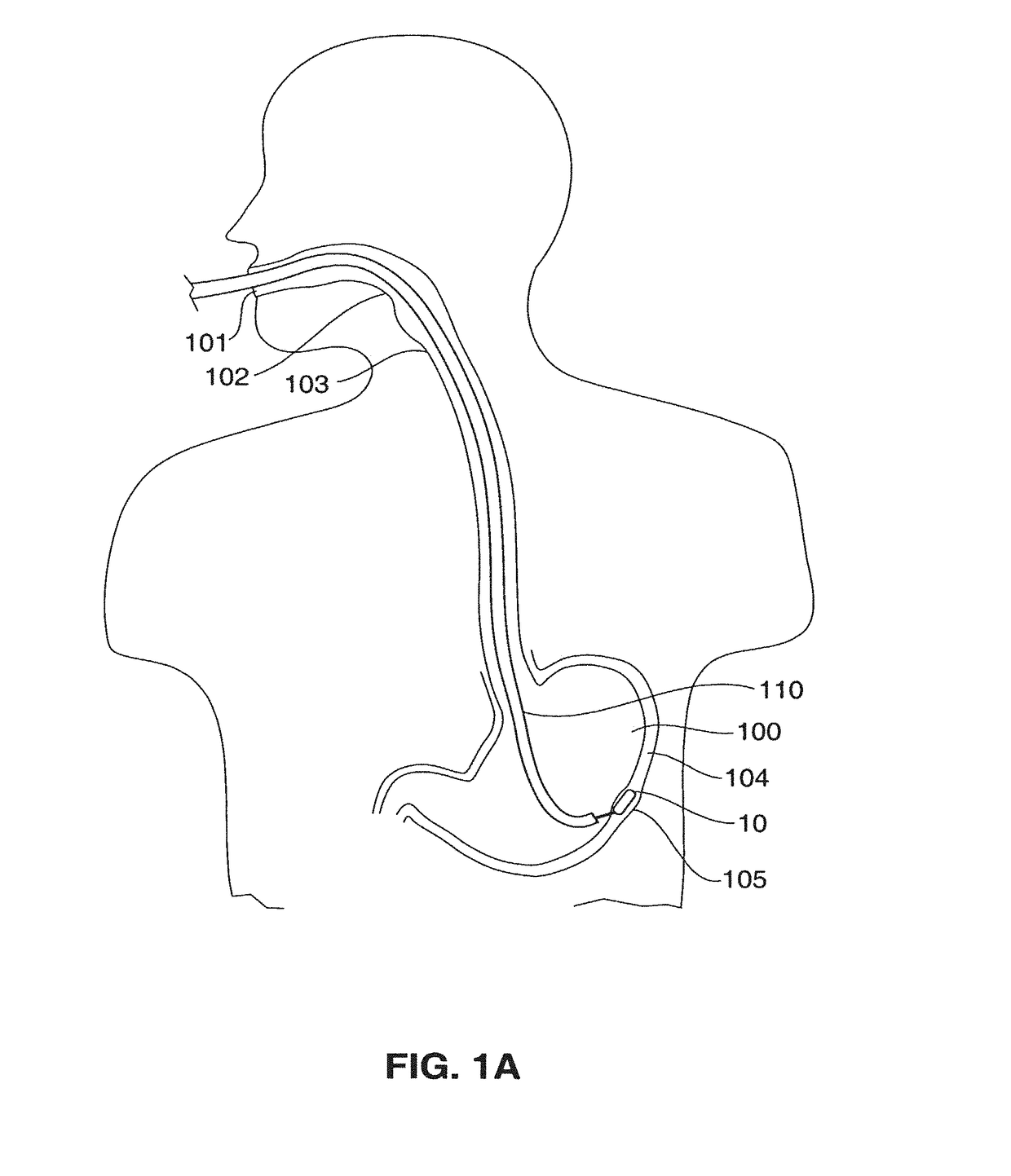
[0107]Referring to FIGS. 2A-2B, a stimulator 10 of a first embodiment is illustrated. The stimulator 10 is constructed in a disc-like shape. The stimulator 10 comprises a housing 20 having a relatively flat, broad top surface 21 with surface electrodes 11, 12 and a sensor 13 located thereon. The diameter d of the top surface is greater in comparison to the height h of the side (FIG. 2B) of the stimulator 10. The disc shape maintains the device in proper orientation so that the electrodes 11, 12 contact the muscle layer 104c of the stomach wall 104. FIG. 2B illustrates an aspect 15 of all side views of the stimulator 10. The aspect ratio of the aspect 15 is the width of the side view (diameter d) divided by the height h of the side view, and is greater than about one, preferably greater than about 1.4 and more preferably greater than about 1.8.
[0108]When implanted the electrodes 11, 12 are oriented so that they face and are in electrical contact with the muscle layer 104c of the stom...
second embodiment
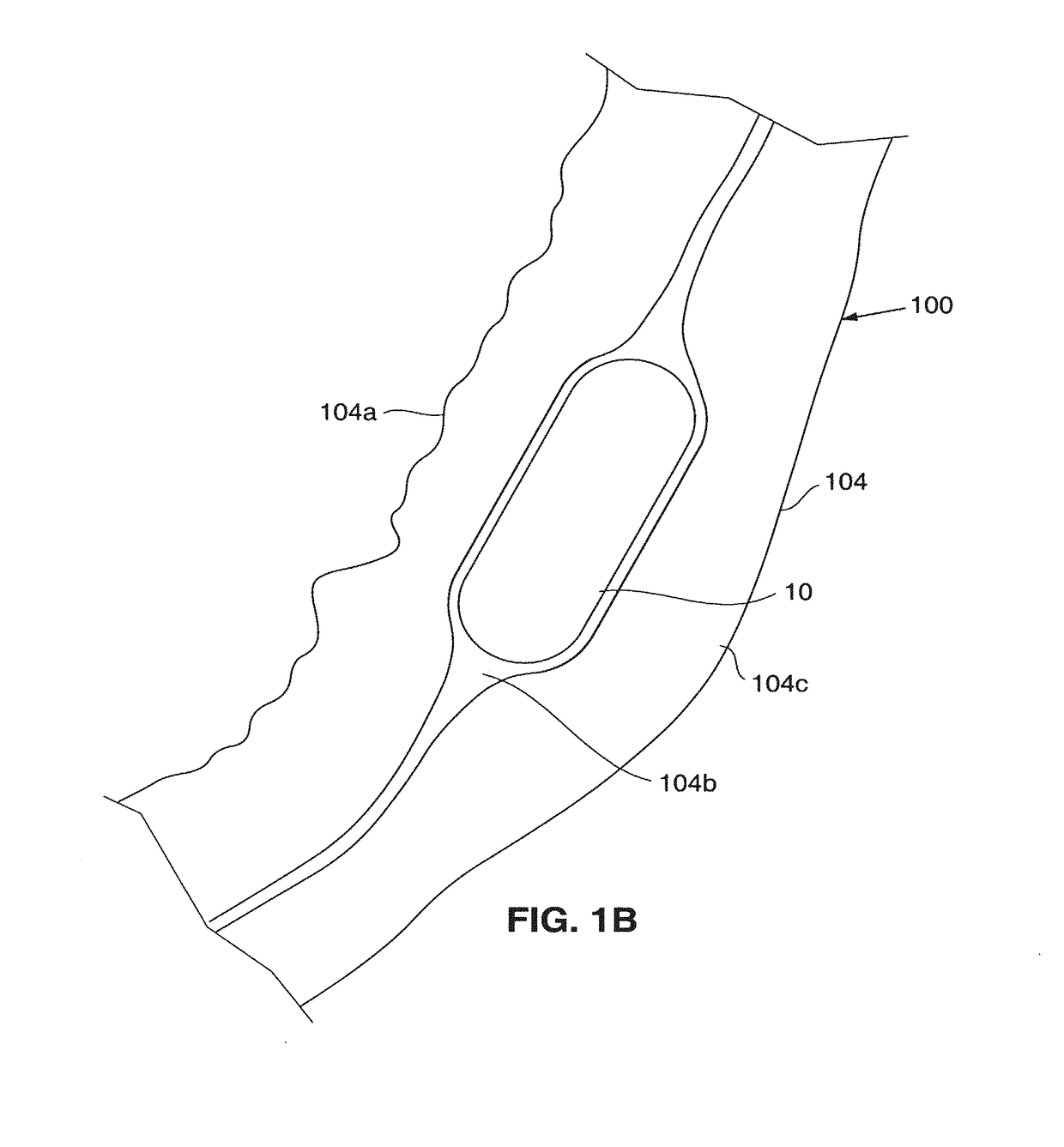
[0163]Referring now to FIGS. 18A-E a second embodiment is illustrated in which a balloon is used to dissect the connective tissue in the submucosal layer 104b. A hollow endoscopic needle 230 containing a balloon tipped instrument 231 having a compliant balloon 232 on the distal end of the instrument 231, is placed at the opening 201 (formed in the mucosal wall 104a when the bleb 200 is formed (FIG. 14A)), and into the submucosal layer 104b (FIG. 14B). The endoscopic needle 230 is retracted leaving the balloon 232 at the opening 201 within the submucosa 104b (FIG. 14C). The balloon 232 is inflated by introducing an inflation medium through the inflation lumen 233 in the instrument 231. (FIG. 14D). The opening 201 is relatively small so as to prevent the balloon 232 from exiting the submucosa 104b when the balloon 232 is inflated. As it is inflated, the balloon 232 expands distally as well as radially to dissect the submucosal tissue. The balloon 232 in this embodiment made of a compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com