Anti-Inflammatory Peptides and Composition Comprising the Same
a technology of anti-inflammatory peptides and compositions, applied in the direction of peptide sources, transferases, immunological disorders, etc., can solve the problems of increased capillary permeability and concentration, increased blood flow rate, and abnormal biological defense response excessively,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of PEP-1 and Measurement of Anti-Inflammatory Activities of PEP-1 (SEQ ID NO: 1)
Experiment 1. Synthesis of PEP-1 (SEQ ID NO: 1)
[0109]A peptide comprised of 16 amino acids with the chemical structure 1 as below having the sequence SEQ ID: 1 (PEP-1) derived from human telomerase was synthesized:
[0110]SEQ ID NO: 1 (PEP-1) was synthesized according to the existing method of solid phase peptide synthesis. In detail, the peptides were synthesized by coupling each amino acid from C-terminus through Fmoc solid phase peptide synthesis, SPPS, using ASP485 (Peptron, Inc., Daejeon ROK). Those peptides with their first amino acid at the C-terminus being attached to resin were used as follows:[0111]NH2-Lys(Boc)-2-chloro-Trityl Resin[0112]NH2-Ala-2-chloro-Trityl Resin[0113]NH2-Arg(Pbf)-2-chloro-Trityl Resin
[0114]All the amino add materials to synthesize the peptide were protected by Fmoc at the N-terminus, and the amino acid residues were protected by Trt, Boc, t-Bu (t-butylester), Pbf (...
experiment 2-1
sis
[0129]The level of nitric oxide (NO) was measured in Raw 264.7 cell (1×106 cell / ml) using Griess reagent system (Promega, USA). Culture medium of 50 μl was added to a 96-well plate and Griess reagent I (NED) solution and Griess reagent II (Sulfanilamide solution) are added in the same amount. After 10 min incubation of cells with the reagents, the optical density at 540 nm was measured within 30 min using a microplate reader (Molecular Devices, USA). The concentration of NO was calculated by using a standard curve (0˜100 μM) of sodium nitrite.
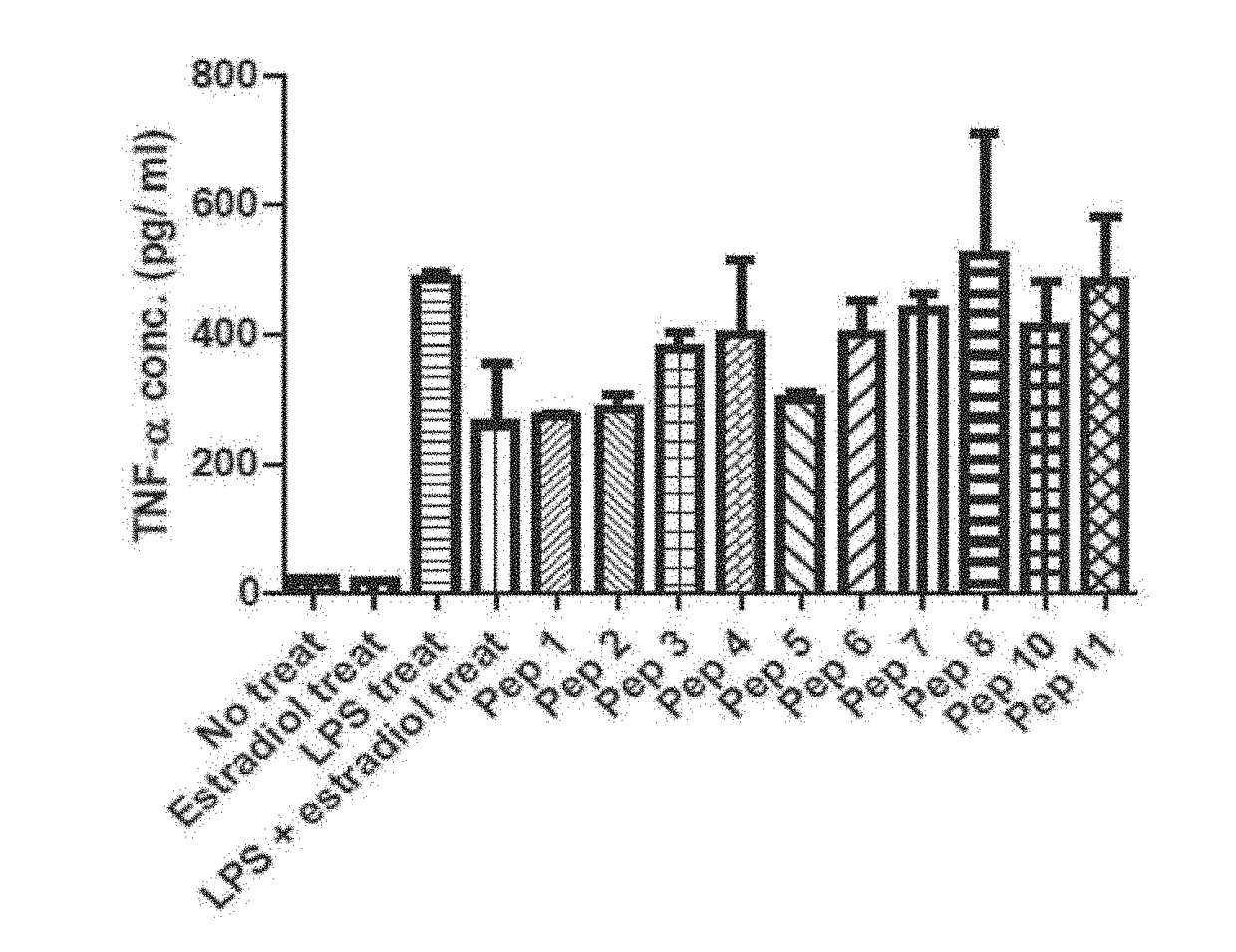
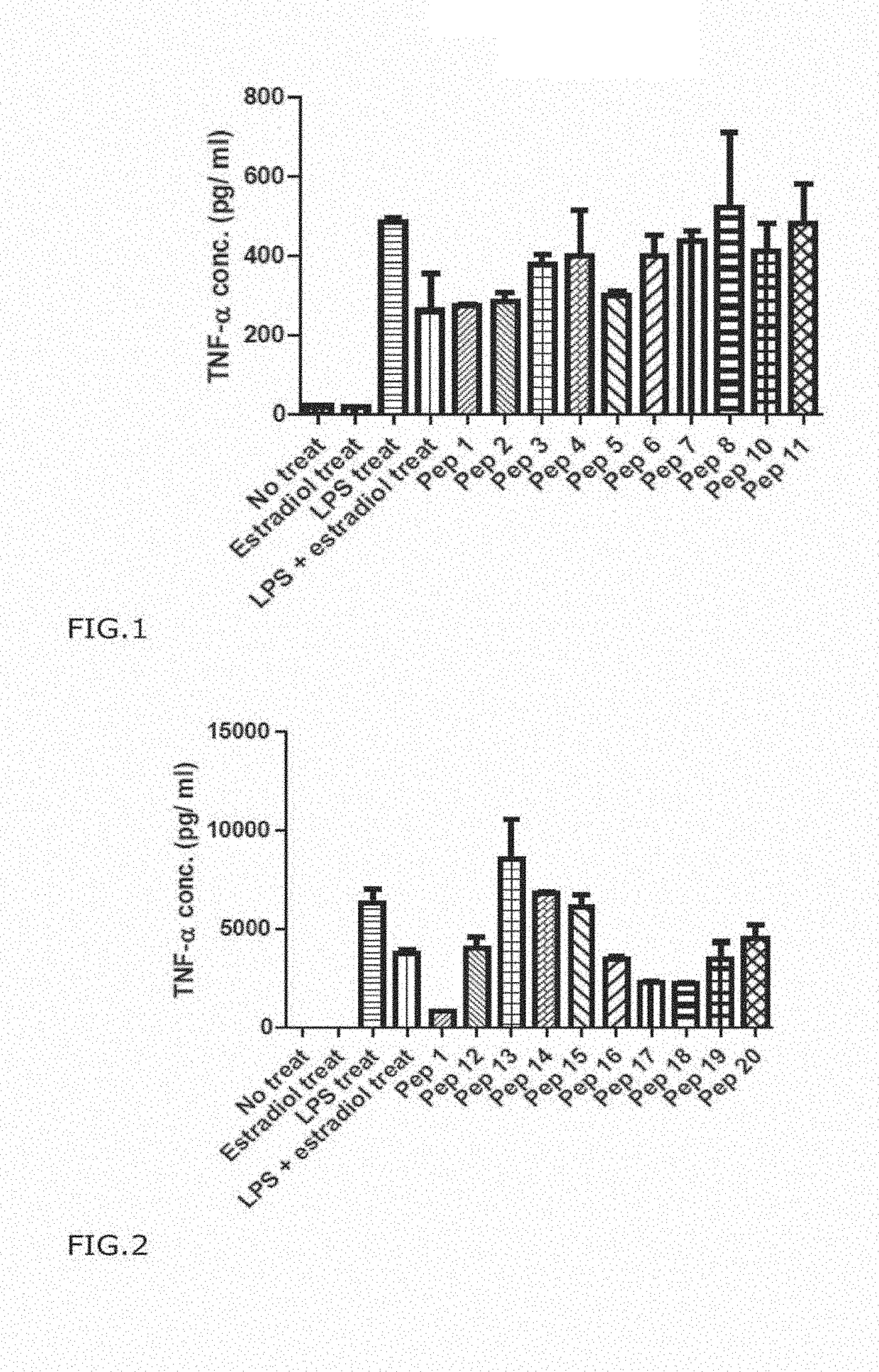
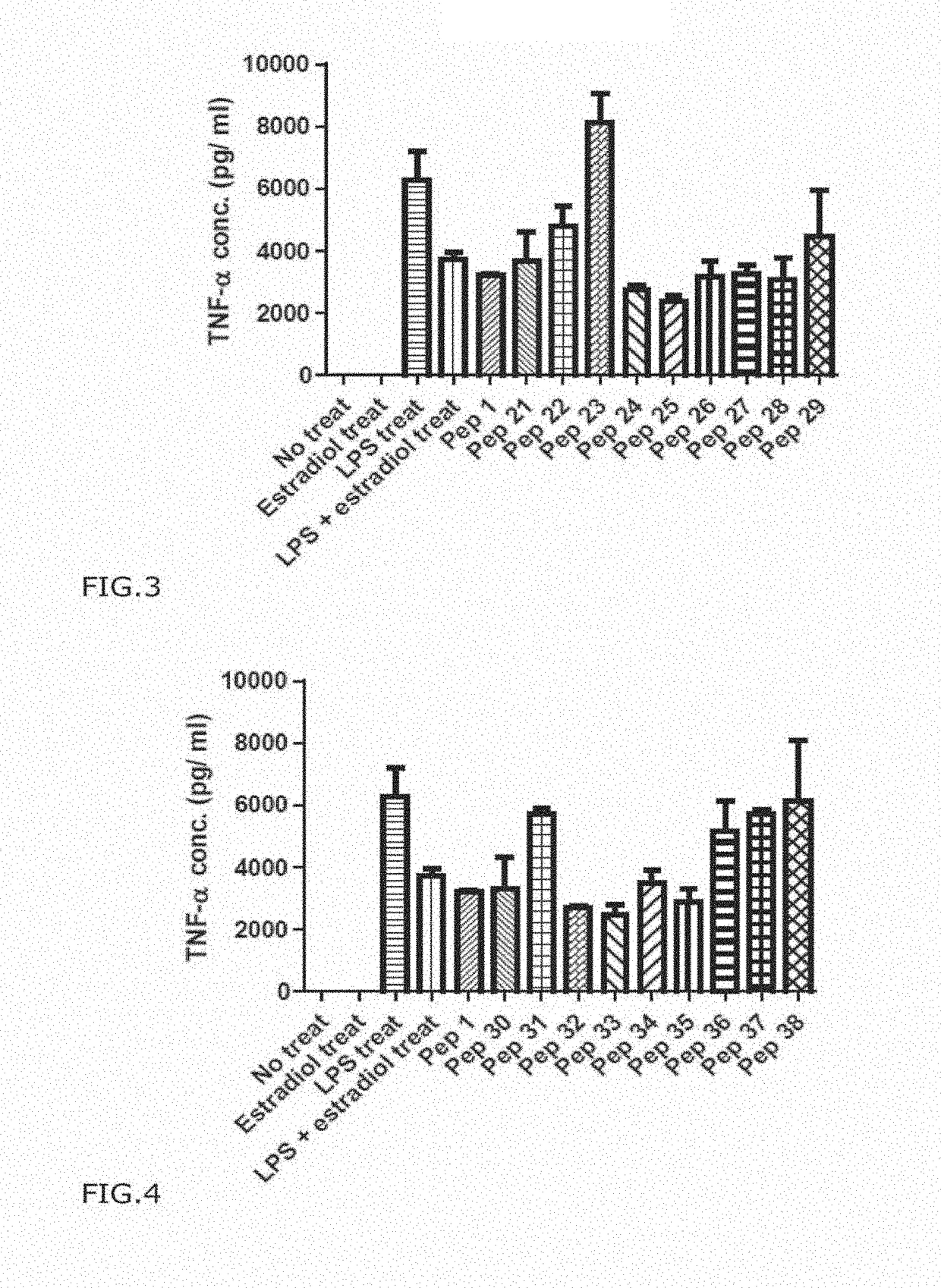
[0130]As shown in Table 3 below, stimulation of cells with LPS increased the expression of NO, but in co-treatment with LPS and Pep1, the expression level of NO mentioned above decreased. NO is produced during inflammation, and the result showing Pep1 reduced NO level to 65% of the control strongly support the anti-inflammatory effect of Pep1.
TABLE 3The measurement of anti-inflammatory effect of humantelomerase derived PEP 1NO ExpressionDecr...
experiment 2-2
tokine Inhibitory Effect
[0131]To investigate the effect of PEP1 on inhibiting pro-inflammatory cytokine production RAW 264.7 cell were pre-treated with PEP 1 at a concentration of 5 μg / mL challenged with LPS at a concentration of 1 μg / mL, and cells were further incubated for 24 hr. The supernatant samples containing cell culture medium was collected and analyzed for the cytokine levels using ELISA kits (eBloscience, San Diego).
[0132]96 wells plates were coated with 100 μl of capture antibodies (diluted in coating buffer to the concentration recommended by manufacturer's protocol) overnight at 4° C. Then, after washing the plates 5 times, 200 μL of assay diluents was added to each well and incubated for 1 hr at room temperature for blocking. After washing each well with wash buffer five times, cell culture sample or each cytokine standard protein sample was diluted and 100 μl of each added into each well. The plate containing samples were incubated overnight at 4° C.
[0133]Then, after...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com