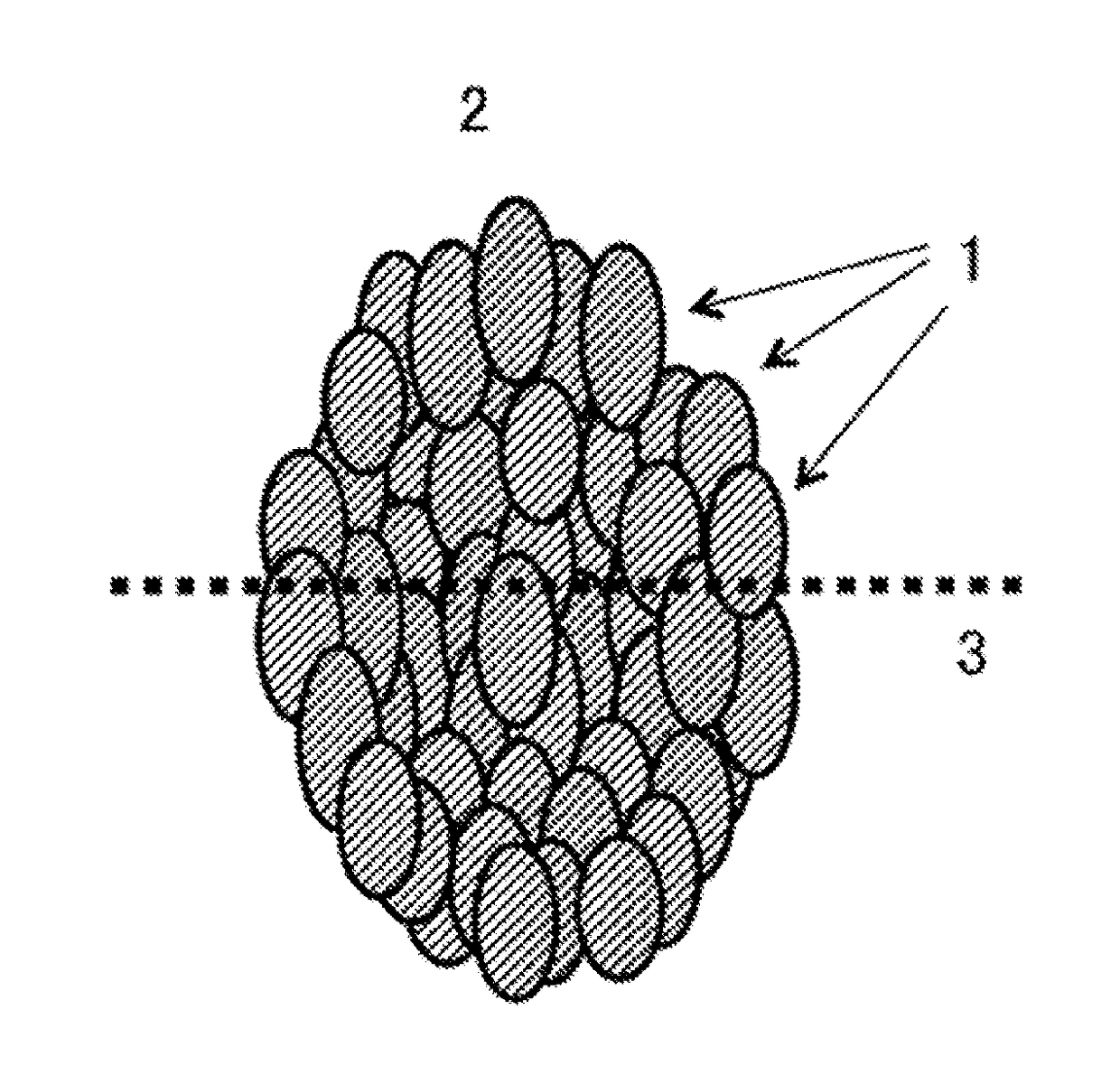
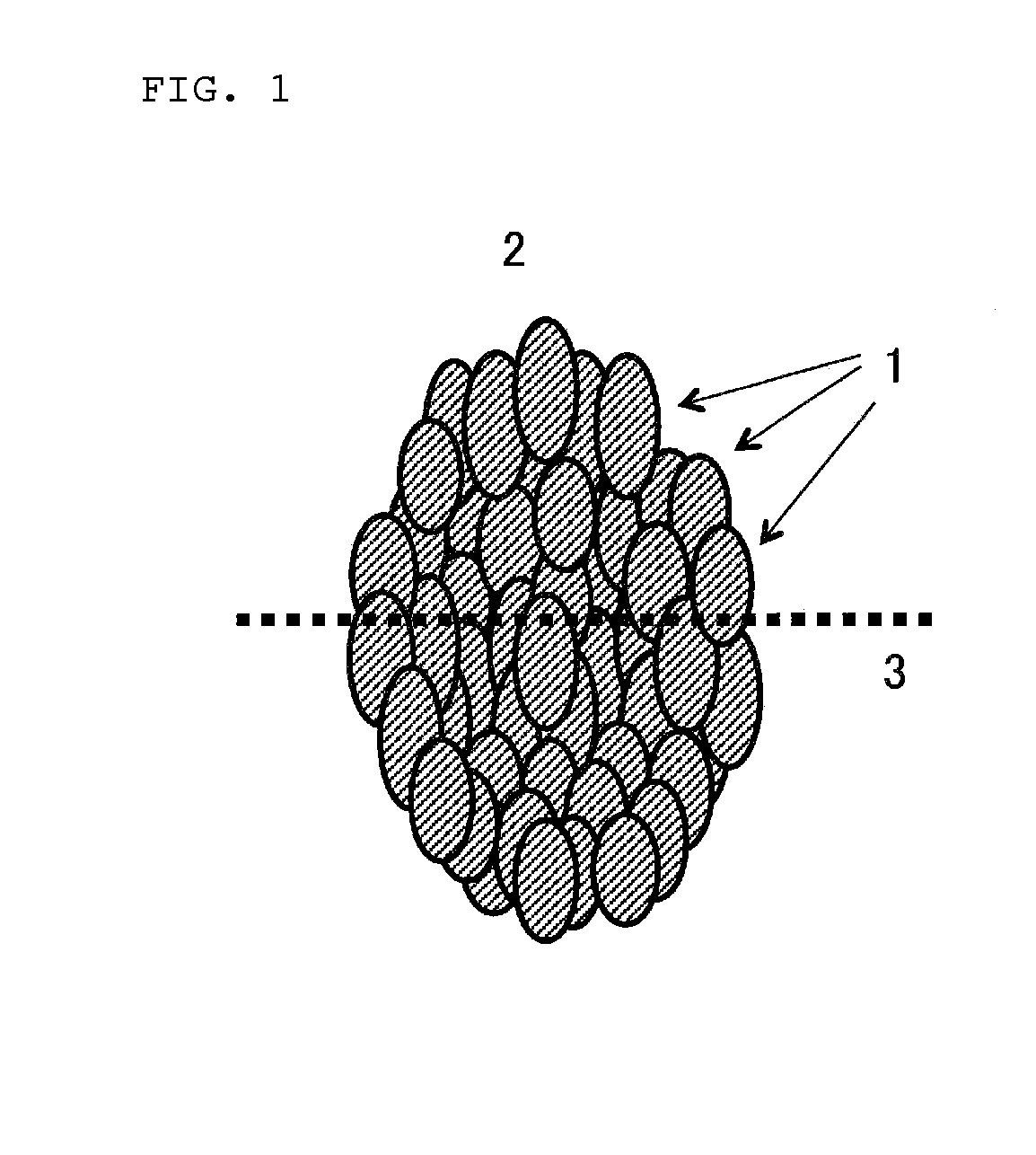
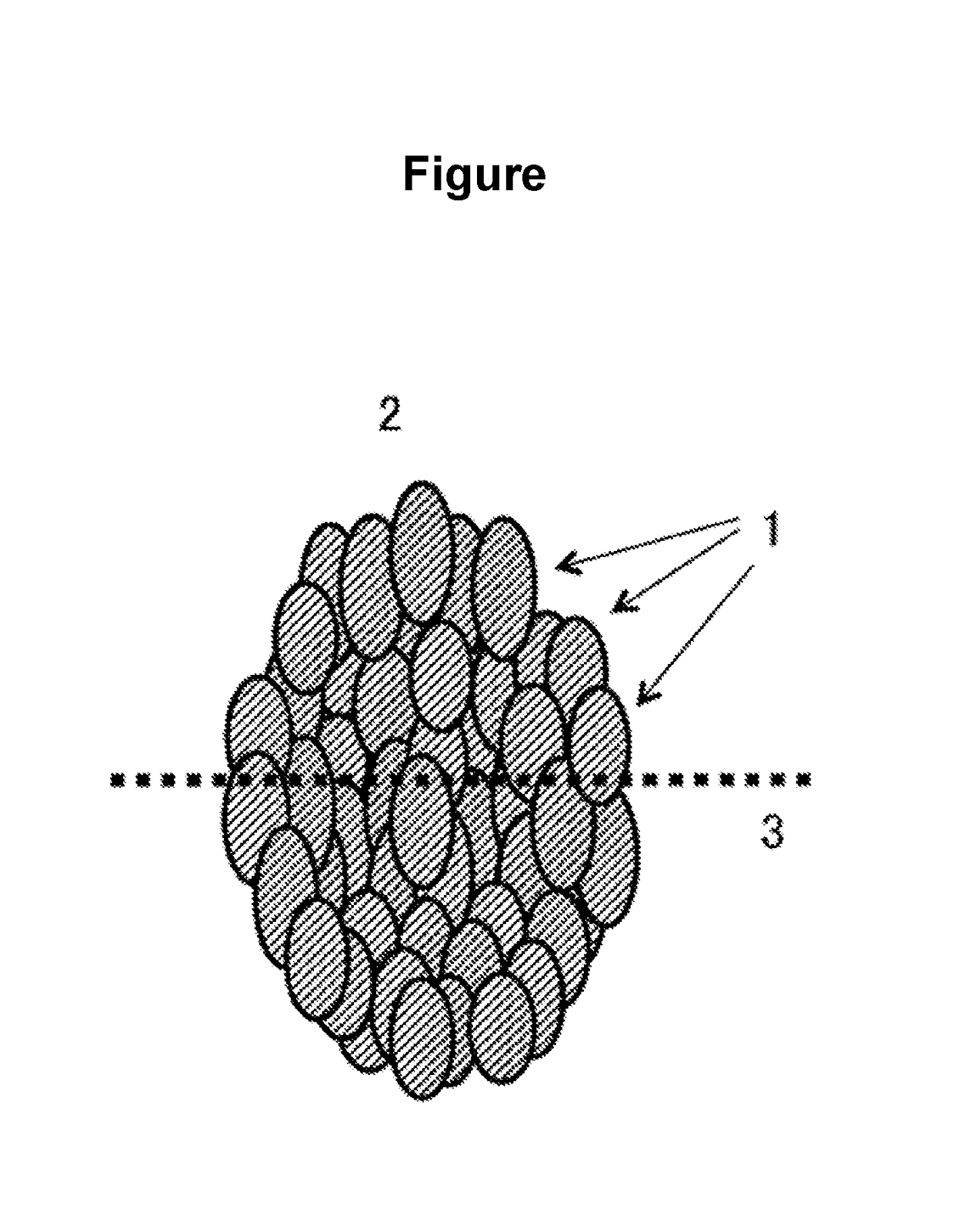
Positive electrode active substance for non-aqueous electrolyte secondary batteries, and non-aqueous electrolyte secondary battery
a technology of active substances and positive electrodes, which is applied in the direction of cell components, electrochemical generators, and nickel compounds, etc., can solve the problems of li concentration variation the literature 1 to 3 fails to specifically describe the variation of li concentration in grain boundary and crystal variation, etc., to improve the output characteristics and durability of active substances, reduce conductive path or deterioration, and good effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0067]In a reaction vessel equipped with a blade-type stirrer, a sodium hydroxide aqueous solution having a pH value of 12.0 was prepared, and an ammonia aqueous solution was added dropwise into the sodium hydroxide aqueous solution such that the obtained reaction solution had an ammonia concentration of 0.80 mol / L. Furthermore, a mixed solution comprising cobalt sulfate, nickel sulfate and manganese sulfate was continuously fed to the reaction vessel. During the aforementioned procedure, a sodium hydroxide aqueous solution and an ammonia aqueous solution were continuously fed to the reaction vessel so as to control a pH value of the resulting reaction solution to 12 and an ammonia concentration thereof to 0.8 mol / L, so that the particles in the reaction solution were grown to those having an average secondary particle diameter as aimed, and further by applying a mechanical shear force to the resulting suspension, a precipitate comprising a spherical composite transition metal was o...
example 2
[0073]The same procedure as in Example 1 was conducted except that the ratio of Ni / Co / Mn was changed to 1.0 / 1.0 / 1.0, and a mixture comprising the Li raw material and the transition metal mixed spherical oxide was calcined in an oxygen atmosphere at 750° C. for 10 hours, and then the resulting calcined product was deaggregated to produce positive electrode active substance particles, thereby obtaining a positive electrode active substance.
[0074]The section of the thus obtained particles was subjected to Nano-SIMS element distribution analysis, so that it was confirmed that a coefficient of variation of Li / Me in the composition including the crystals and grain boundaries was 18.7%.
[0075]As a supplemental measurement, using high resolution TEM, multi-wave interference images and selected area electron diffraction patterns as well as STEM-EELS analysis were conducted from the grain boundaries to an inside of the crystals at intervals of 20 nm. As a result, it was confirmed that the crys...
example 3
[0077]The same procedure as in Example 2 was conducted except that the ratio of Ni / Co / Mn was changed to 1.0 / 1.0 / 1.0, and the ratio of Li / Me was changed to 1.00 (Li / Me=1.00), thereby obtaining a positive electrode active substance.
[0078]The section of the thus obtained particles was subjected to Nano-SIMS element distribution analysis, so that it was confirmed that a coefficient of variation of Li / Me in the composition including the crystals and grain boundaries was 7.1%.
[0079]As a supplemental measurement, using high resolution TEM, multi-wave interference images and selected area electron diffraction patterns as well as STEM-EELS analysis were conducted from the grain boundaries to an inside of the crystals at intervals of 20 nm. As a result, it was confirmed that the crystal structure in the vicinity of the grain boundaries was the same R-3m structure as that of a bulk thereof, and no reduction of the transition metals was caused.
[0080]The resulting positive electrode active subst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| crystallite size | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com