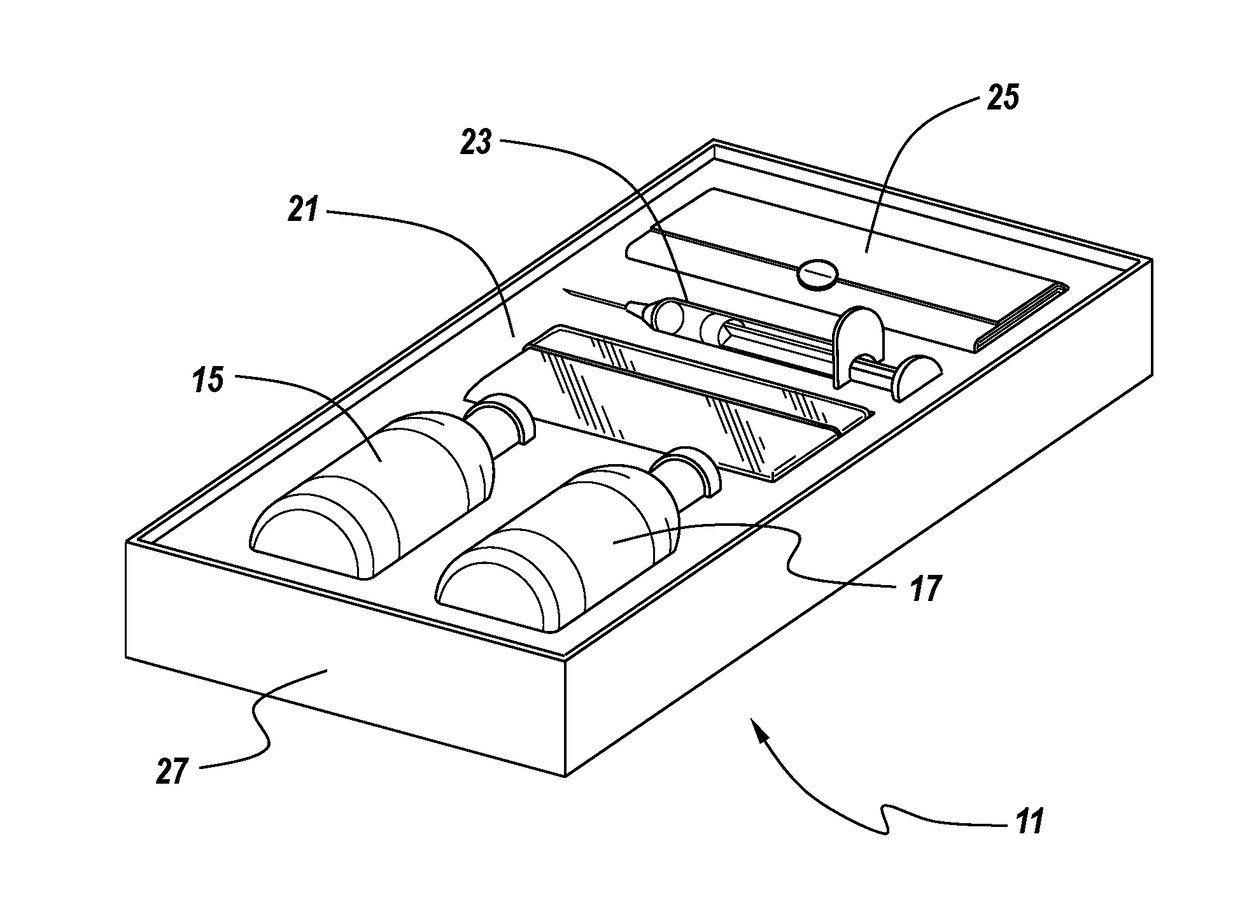
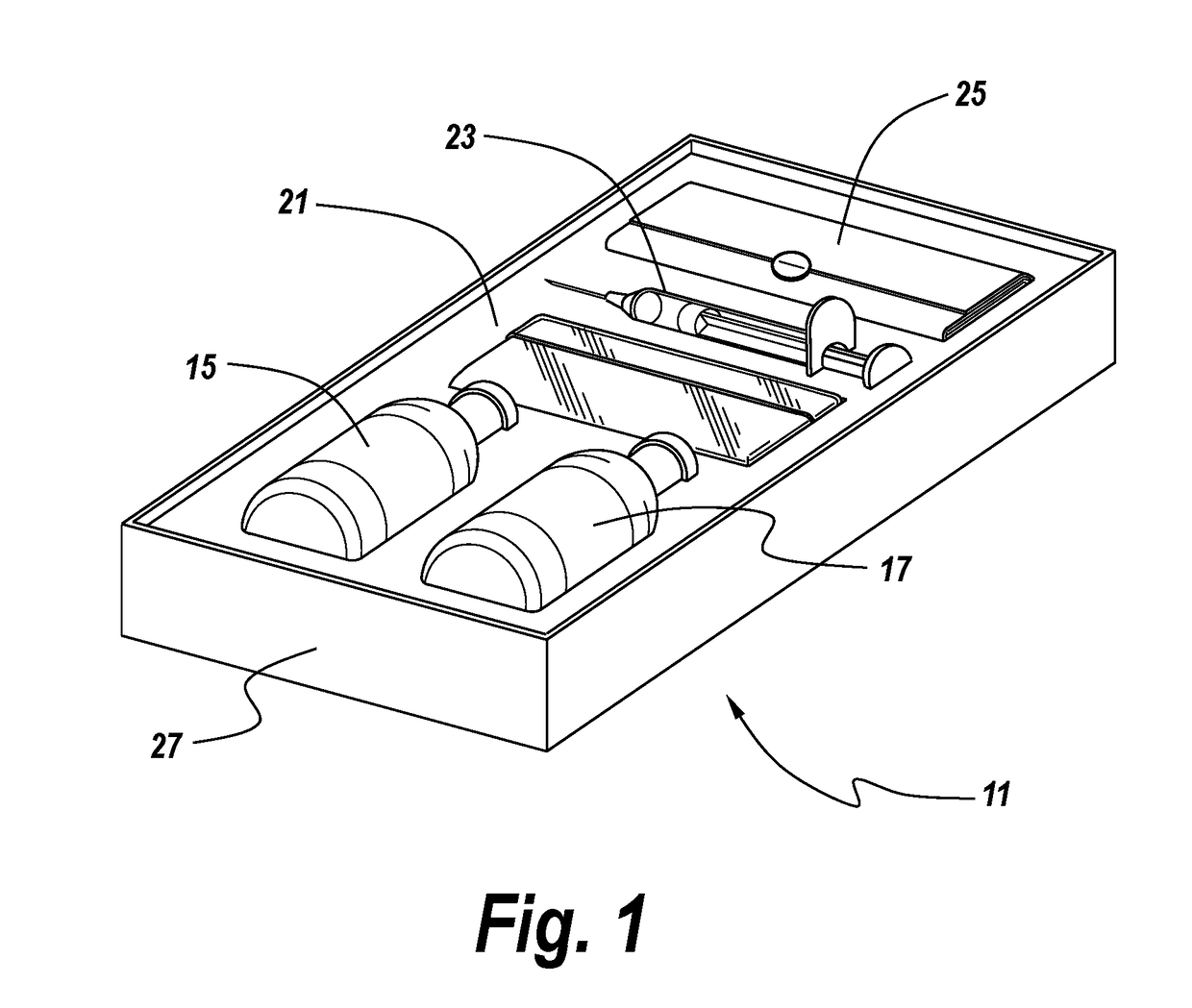
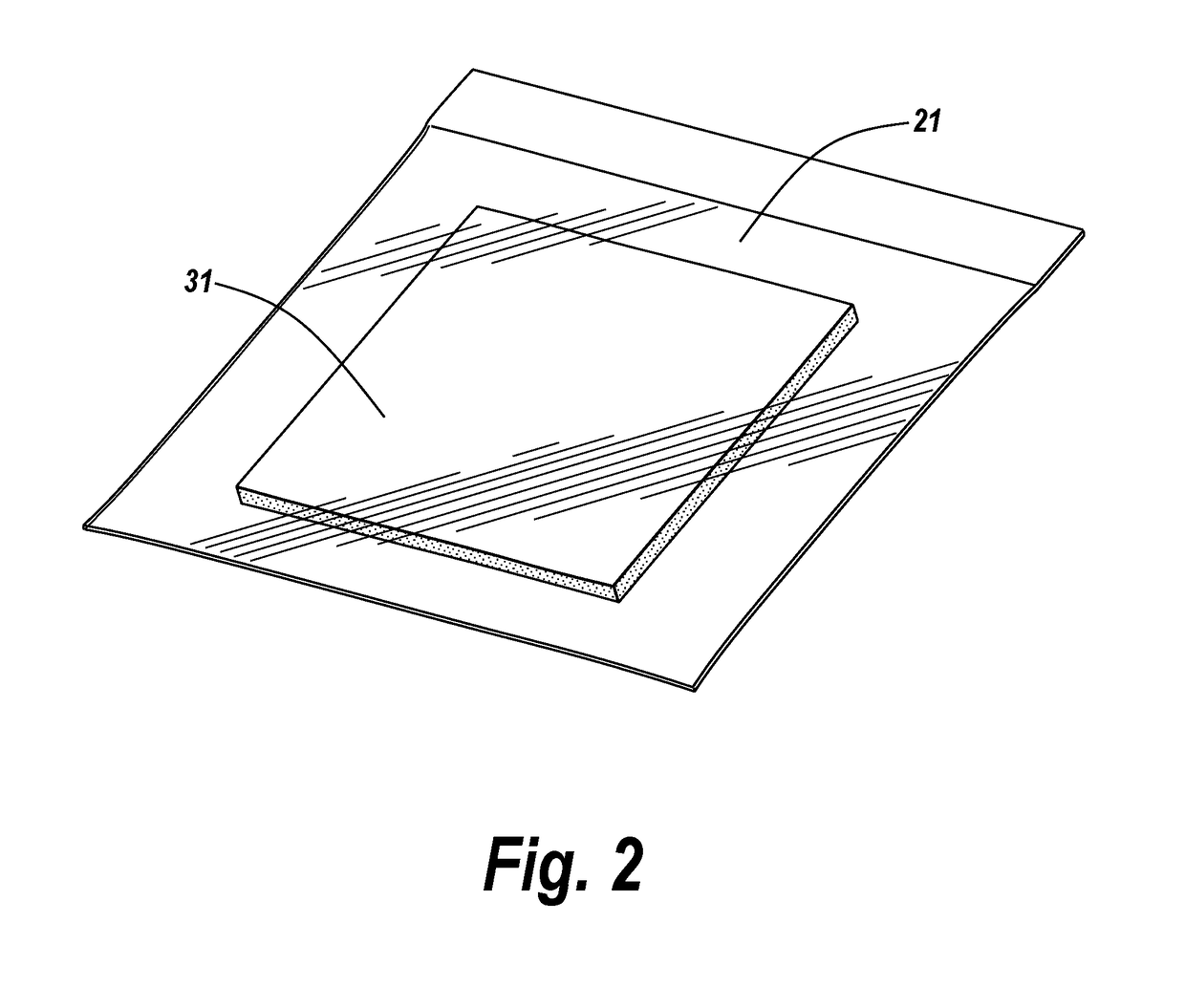
Methods and articles of manufacture for the treatment of animals
a technology of animal and article manufacture, applied in the field of methods and articles of manufacture for the treatment of animals, can solve the problems of animal and human injuries that are difficult to treat, animal or individual in jeopardy with potential loss of function, failure to adequately, etc., and achieve the effect of promoting healing and promoting healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Material Processing
[0078]This procedure defines the aseptic collection of amniotic material (amnion and amniotic fluid) for injection at the site of an injury.
[0079]Amnion Tissue
[0080]The amnion container was picked up and sampled for Bioburden. The amnion was aseptically transferred into the sterile field (laminar flow hood). The amnion transport packaging (previously disinfected, i.e. with 70% ETOH) was opened.
[0081]A 50 mL sample of the Amnion Transport Solution was aseptically transferred into a 30 to 60 mL conical tube for pre-processing bioburden testing. The vial was labeled with sample description, batch number, date and time and placed in a designated refrigerator.
[0082](1) Amnion Preparation
[0083]The amnion from the incoming container was transferred into approximately 200 mL of Plasma Lyte-A in a sterile bioassay dish where it was gently rinsed. A piece of amnion was then spread evenly on a sterile cutting board carefully avoiding any overlaps. A record was made of the am...
example 2
rocessing of the Amniotic Material
[0093](1) Aseptic Cryofractionation of Amnion
[0094]After at least one hour, the amnion was removed from the drying rack and transferred into the milling chambers having an impactor. The milling chambers were placed into the Cryomill and cryofractionated using the following settings:
[0095]Number of Cycles: 4
[0096]Frequency 1 / s: 10 CPS—
[0097]Precooling Time: 10 minutes
[0098]Grinding Time: 4 minutes
[0099]Intermediate Cooling: 3 minutes
[0100]Once grinding was complete, the milling chambers were allowed to warm to room temperature for approximately two hours. The start and stop times were recorded.
[0101]Approximately 50 mL of the amnion suspension solution was dispensed into each milling chamber. The inside milling chamber and the impactor were rinsed with the solution multiple times until the ground amnion (dried particulate mixture) was re-suspended and collected in the bottom of the chamber. The impactor was removed using the magnet pen. The cryofract...
example 3
Tissue Wrap Preparation
[0111]After confirming the amniotic tissue source and donor mare ID and recording the time of receipt, the amnion transport packaging (previously disinfected, i.e. with 70% ethanol, methanol, etc.) was aseptically transferred into the sterile field (a laminar flow hood). A sample of the Amnion Transport Solution was first transferred into a 50 mL conical tube for Bioburden testing. The vial was then labeled with sample description, batch number, date and time and placed in designated refrigerator.
[0112](1) Amniotic Membrane Wrap Preparation
[0113]Saline was aseptically added into a second receiving pan in the sterile field (i.e. laminar flow hood) and the amnion tissue was taken from incoming receiving pan to the second receiving pan containing the sterile saline. Any remaining blood was rinsed with sterile saline. After documenting the amnion preparation start time, sterile gauze or laps was used to remove any remaining debris / blood from the surface of the amn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com