Method for screening cancer
a cancer and screening method technology, applied in the field of screening cancer, can solve the problems of high cost, difficult promotion, high false positive rate, etc., and achieve the effect of facilitating the determination of abnormal specimens and sensitivity and specificity of the screening method of the present invention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
and Methods
1. Materials
[0054]The tested materials comprise a series of intact cervical lesion samples, including: squamous cell carcinoma (SCC), adenocarcinoma (AC), and normal cervical samples. All the cervical samples (SCC+AC, n=20; normal, n=20), ovary samples (cancer of ovary, n=19; normal, n=14), colon samples (Ca of colon, n=18; normal, n=18), liver tissue samples (HCC, n=18; normal, n=18), oral samples (oral Ca, n=20; normal, n=19), endometrial cancer samples (endometrial Ca, n=20; normal, n=20), breast tissue samples (cancer of breast, n=17; normal, n=17), sarcoma samples (sarcoma, n=18; normal, n=16) are obtained from Tri-Service General Hospital in Taipei. The genomic DNA of each sample is extracted using QIAamp DNA kit followed by DNA modification kit (CpGenome™ DNA Modification Kit, Millipore, Temecula, Calif.) produced by Millipore to perform bisulfite modification for analysis of DNA methylation of the whole genome.
2. Analysis of DNA Methylation Using Whole Genomic Met...
example 2
of methylated target genes
[0061]Screen 14,475 genes using Infinium HumanMethylation27K methylation chips.
[0062](1) Select those having higher methylation rate score (β value >0.4 and <0.4), combine Gene Expression database (GE07803), and analyze gene enrichment (The Database for Annotation, Visualization and Integrated Discovery, DAVID). 92 genes are selected;
[0063](2) Cancer cell lines are treated with 5-AZc and TSA. Analyze the 92 genes using QRT-PCR to confirm that gene expression is influenced by methylation. 61 genes are left;
[0064](3) Mix the samples to be detected and use MSP to analyze DNA methylation of the 61 genes. 26 genes are left;
[0065](4) Analyze DNA methylation in cancer samples for the 26 genes by MSP. 21 genes are left;
[0066](5) Analyze DNA methylation in cancer tissues for the 21 genes by MSP. 16 genes are left;
[0067](6) Analyze DNA methylation in cancer samples for the 16 genes by Q-MSP. 14 genes are left.
[0068]Confirm the methylation status of genes by pyroseque...
example 3
on Analysis of Target Genes in Cervical Cancer Samples
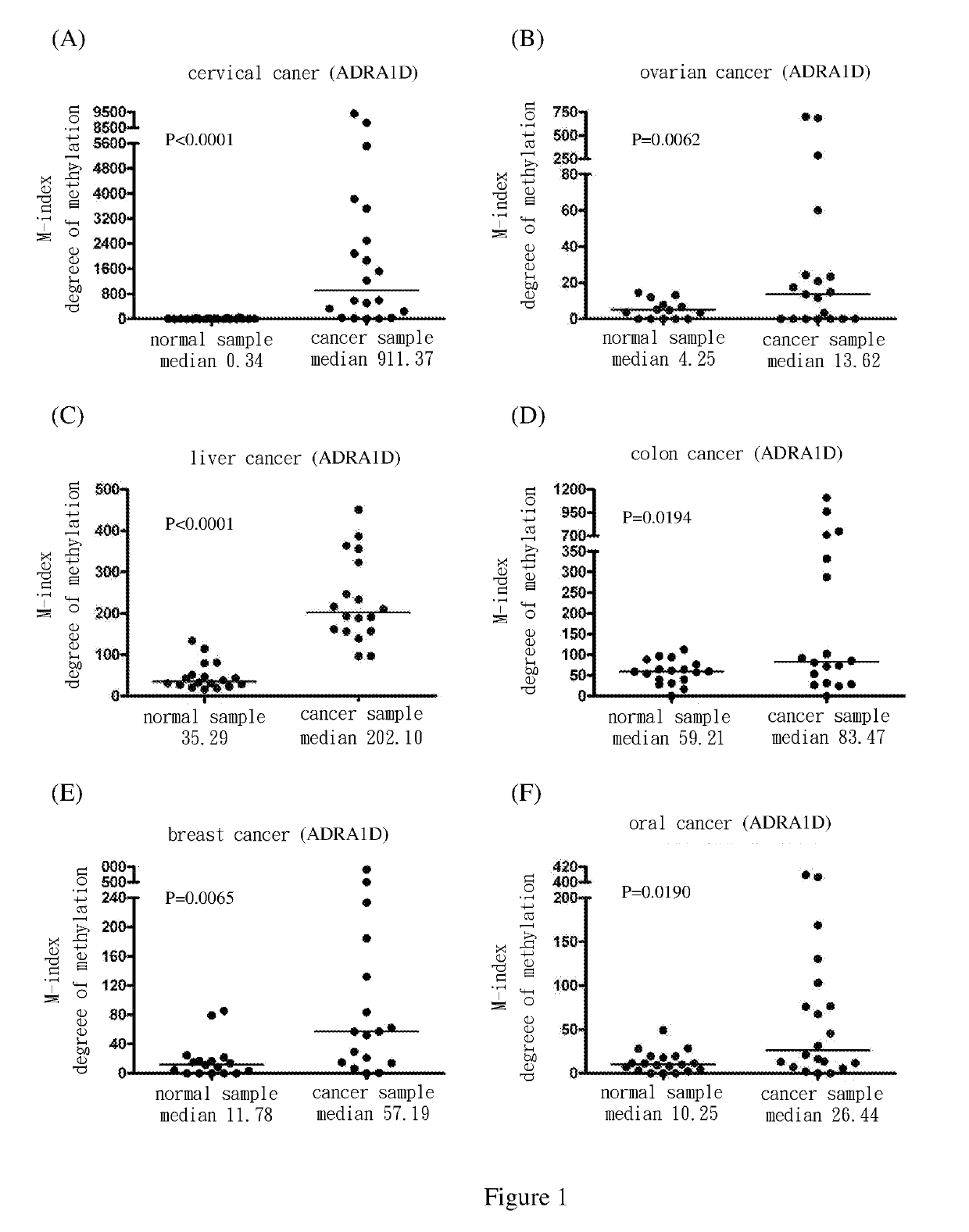
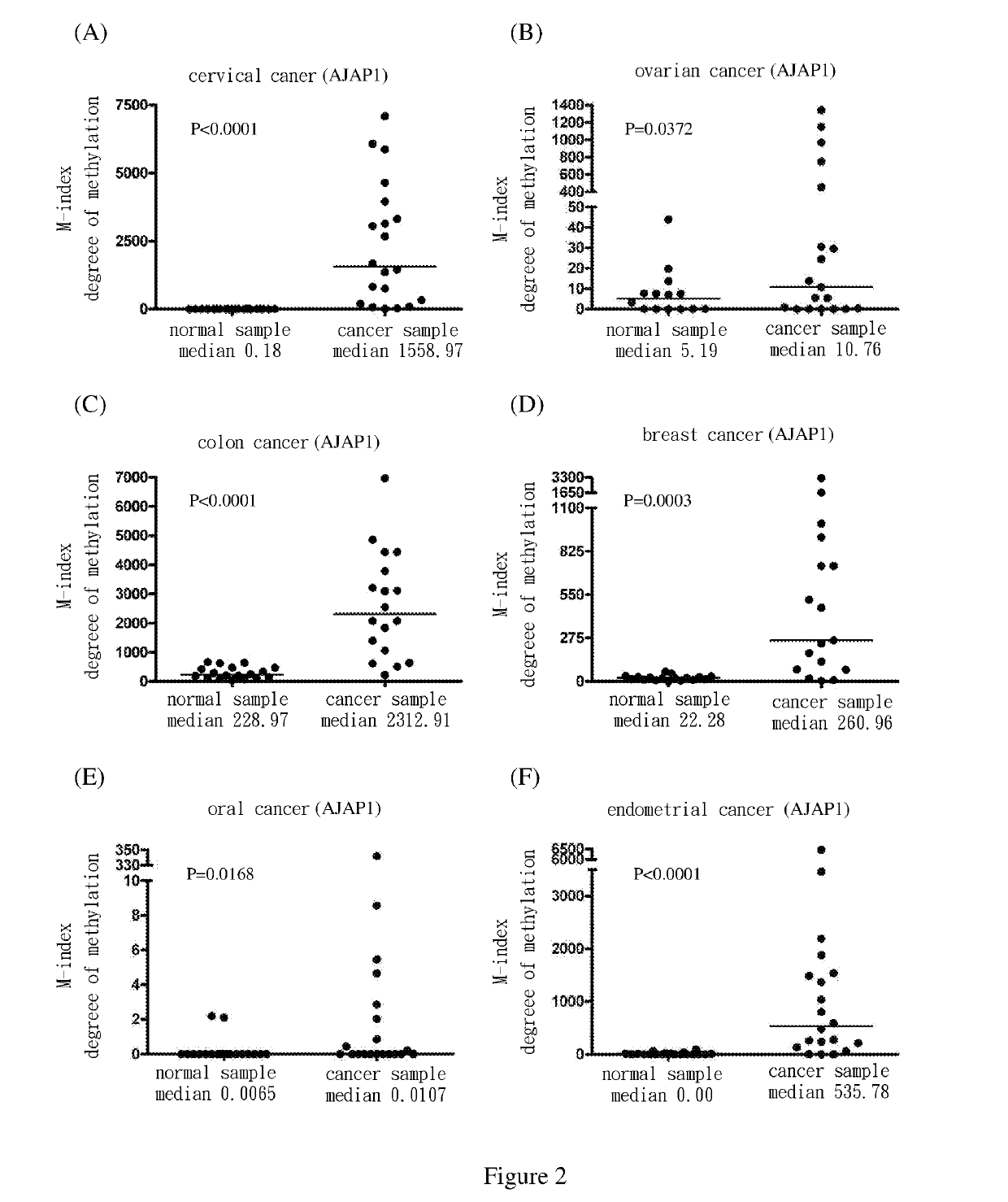
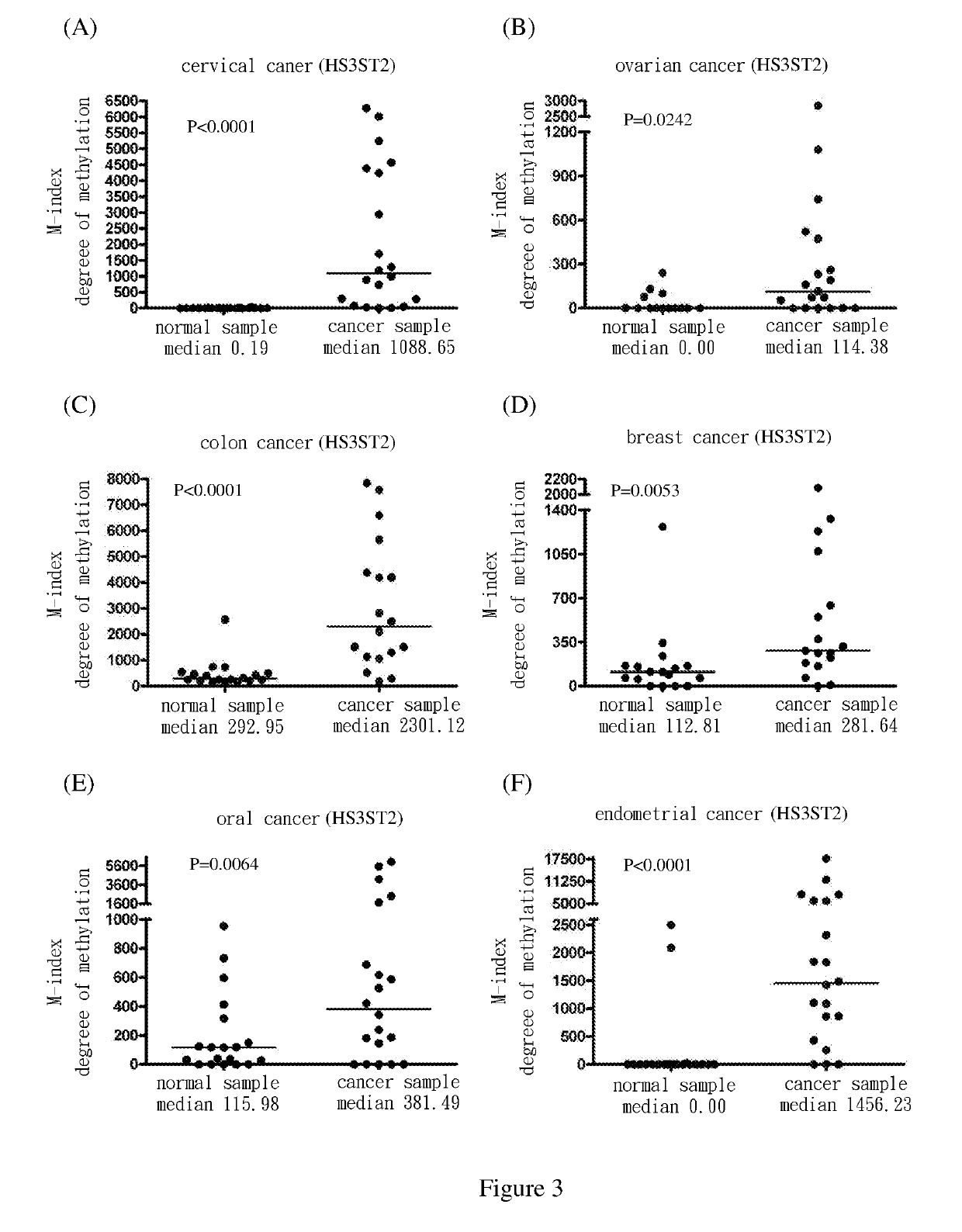
[0069]Use methylation specific PCR (MSP) to analyze the methylation status of the nine target genes in cervical squamous cell cancer samples. The results show the degree of methylation of ADRA1D, AJAP1, HS3ST2, MAGI2, POU4F2, POU4F3, PTGDR, SOX17 and SYT9 in normal cervical samples (SCC N) (the medians are 0.34, 0.18, 0.19, 2.58, 7.62, 0.77, 0.16, 0.17 and 0.31, respectively); and the degree of methylation of ADRA1D, AJAP1, HS3ST2, MAGI2, POU4F2, POU4F3, PTGDR, SOX17 and SYT9 in cervical cancer samples (SCC T) (the medians are 911.37, 1558.97, 1088.65, 713.92, 535.01, 1552.71, 305.84, 248.29 and 551.84, respectively). After Mann-Whitney test, the two groups of data reach P<0.0001 among each group. The difference is statistically significant. The above are as shown in FIG. 1(A), FIG. 2(A), FIG. 3(A), FIG. 4(A), FIG. 5(A), FIG. 6(A), FIG. 7(A), FIG. 8(A), and FIG. 9(A).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com