Generation of pancreatic endoderm from pluripotent stem cells using small molecules
a technology of pluripotent stem cells and pancreatic endoderm, which is applied in the field of generating pancreatic endoderm from pluripotent stem cells, can solve the problems of inability to fully mature beta cells in vitro, restrict the use of this treatment as a clinical therapy, etc., and achieve the effect of improving the efficiency of differentiation of human ps cells, improving the fraction of nkx6.1/pdx1 double positive cells, and improving the population of pancreatic cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Small Molecules that Induce NKX6.1 / PDX1 Co-Expression
Small Molecules
[0161]Four different libraries were included in the screen; a kinase inhibitor library (Calbiochem #539743), a bioactive lipid library (Enzo Life Sciences #BML-2800), a nuclear receptor ligand library (Enzo Life Sciences #BML-2802) and a phosphatase inhibitor library (Enzo Life Sciences #BML-2834). The compounds within the bioactive library were tested at 1 uM and 0.1 uM. Compounds from the other libraries were tested at 10 uM and 1 uM. In a second candidate based screening approach, small molecules that target the main signalling pathways involved in pancreas development were included.
NKX6.1 / PDX1 Screen
[0162]The library compounds were screened on top of a bFGF based media formulation for making PE (Ameri et al. 2010) (RPMI1640, Gibco #61870; 12% KOSR, Gibco #10828; 0.1% PEST, Gibco #15140; 64 ng / mL bFGF, Peprotech #100-18B).
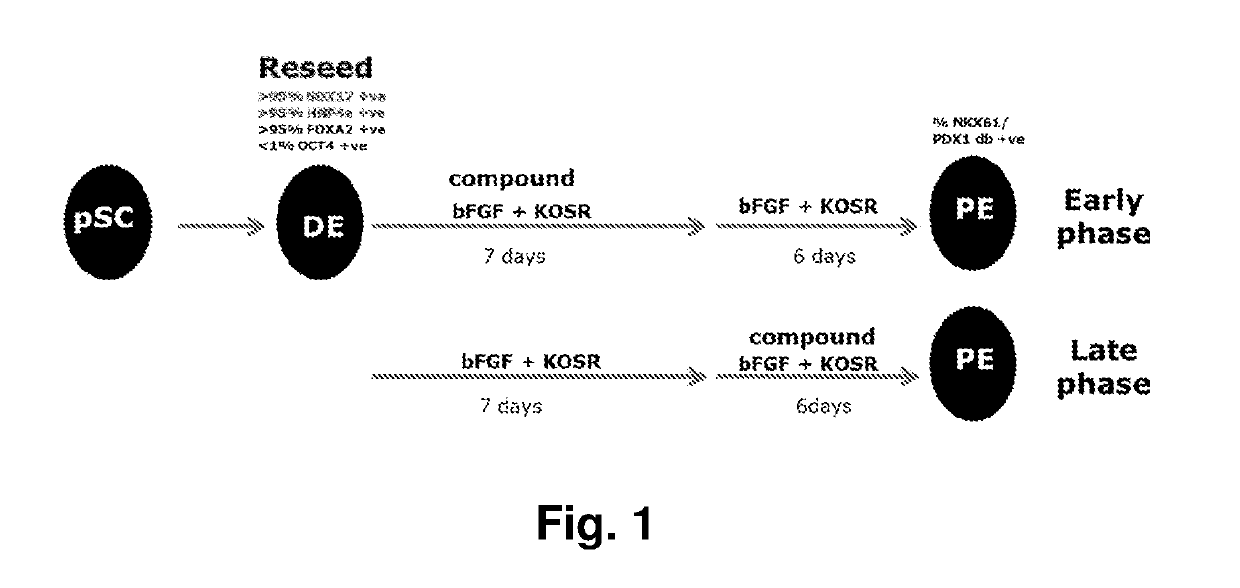
[0163]The library PE screening approach was divided into an early and a late phase (FIG. 1)....
example 2
Small Molecule Hits that Induce NKX6.1 / PDX1 Co-Expression
Combining Hits from the Candidate Screening Approach with Hits from the Library Approach
[0172]DE cells were exposed to 4 days 50 nM LDN-193189, followed by 8 days AM580 (AH Diagnostics, BML GF104 0025), JNK Inhibitor II (Calbiochem, 420119), 50 nM LDN-193189 and 64 ng / ml FGF2, or AM580, JNK Inhibitor II, 50 nM LDN-193189, 64 ng / ml FGF2 and IWP2, or AM580, JNK Inhibitor II, 50 nM LDN-193189, 64 ng / ml FGF2 and Cyclopamine (FIG. 6). Media change was performed daily.
example 3
ion of Small Molecules that Induce NKX6.1 / PDX1 Co-Expression in Human Induced Pluripotent Stem Cells
[0173]Hit compounds (Tables 1 and 2) were screened on top of a bFGF based media formulation for making PE (Ameri et al. 2010) (RPMI1640, Gibco #61870; 12% KOSR, Gibco #10828; 0.1% PEST, Gibco #15140; 64 ng / mL bFGF, Peprotech #100-18B).
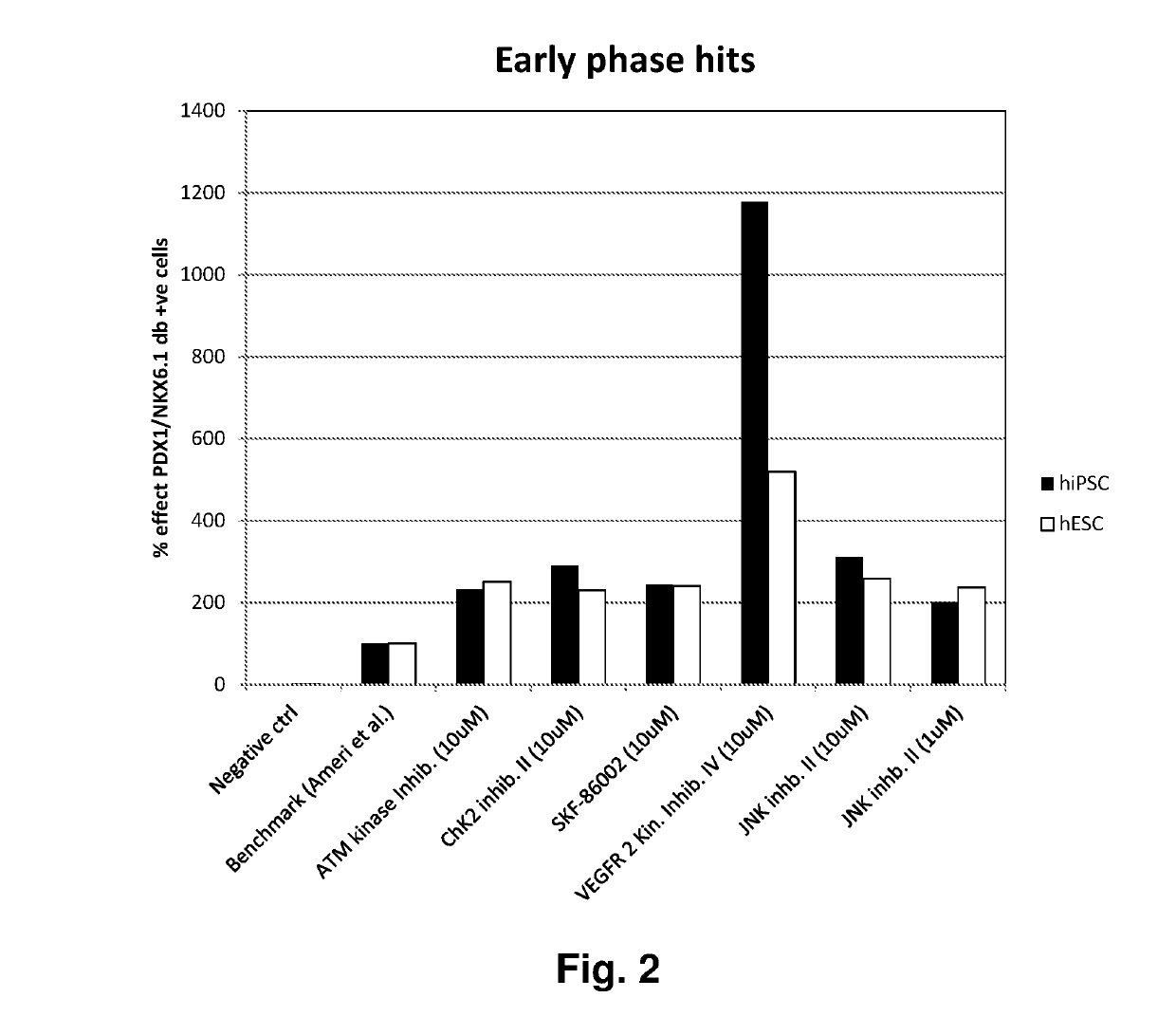
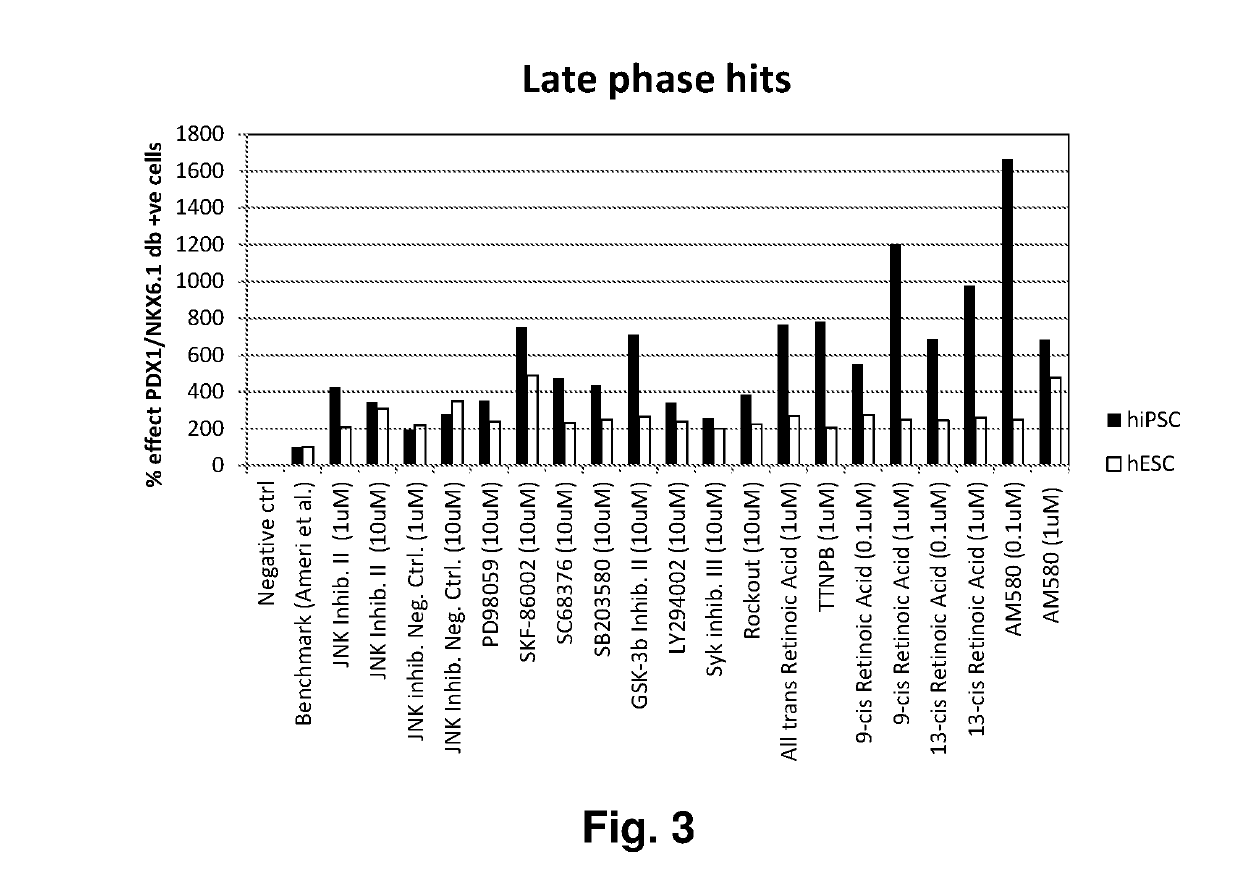
[0174]The screen was divided into an early and a late phase (FIG. 1). In the early phase, compounds were tested on top of the PE media for the first seven days of PE differentiation, and then continued for additional six days using PE media without compounds. In the late phase, DE cells were differentiated in the PE media for the first seven days. In the following six days compounds were tested on top of the PE media. Twelve positive control wells (PE media) and 12 negative control wells (PE media without bFGF) were included in each 96 well plate. Media change was performed daily.
[0175]Values above 200% effect were categorized as a hit (FIG. 2 and FIG. 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com