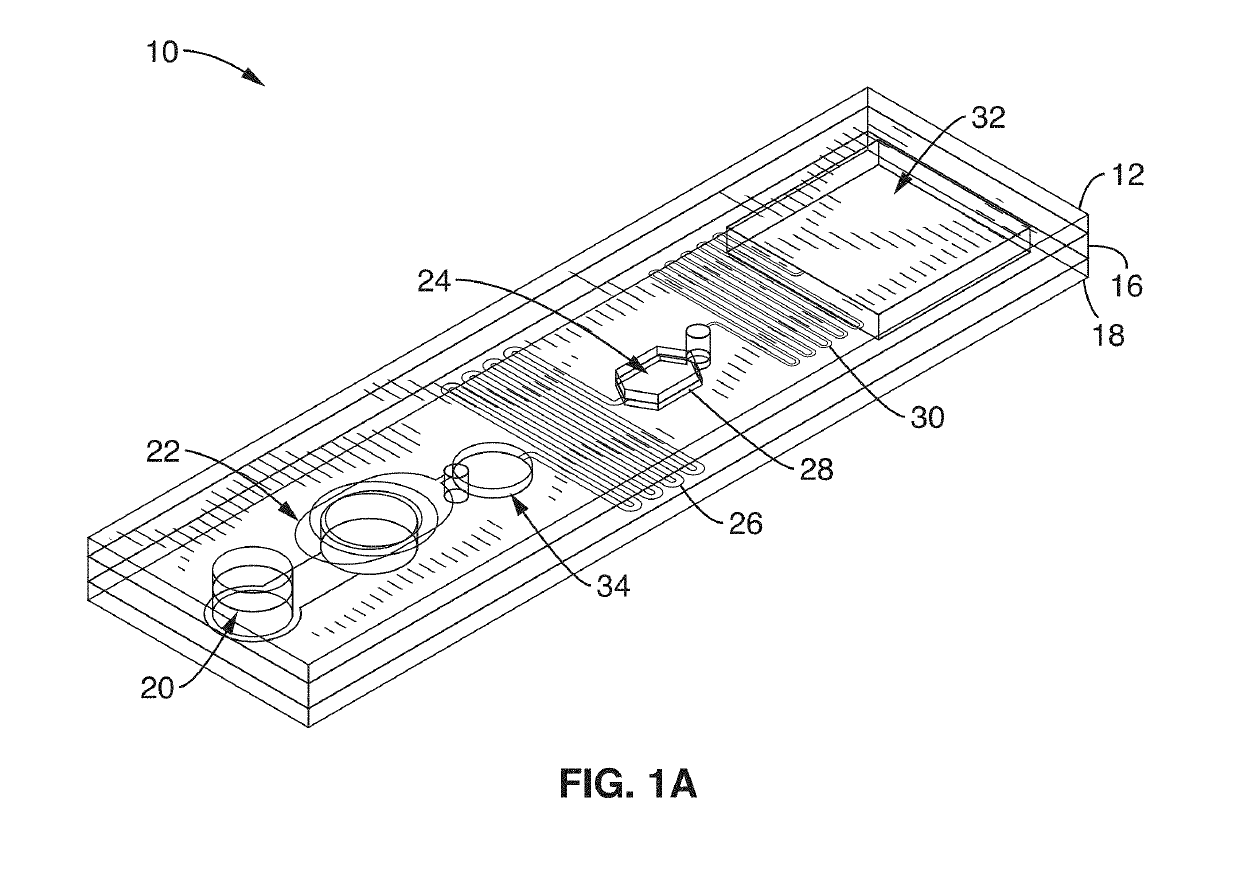
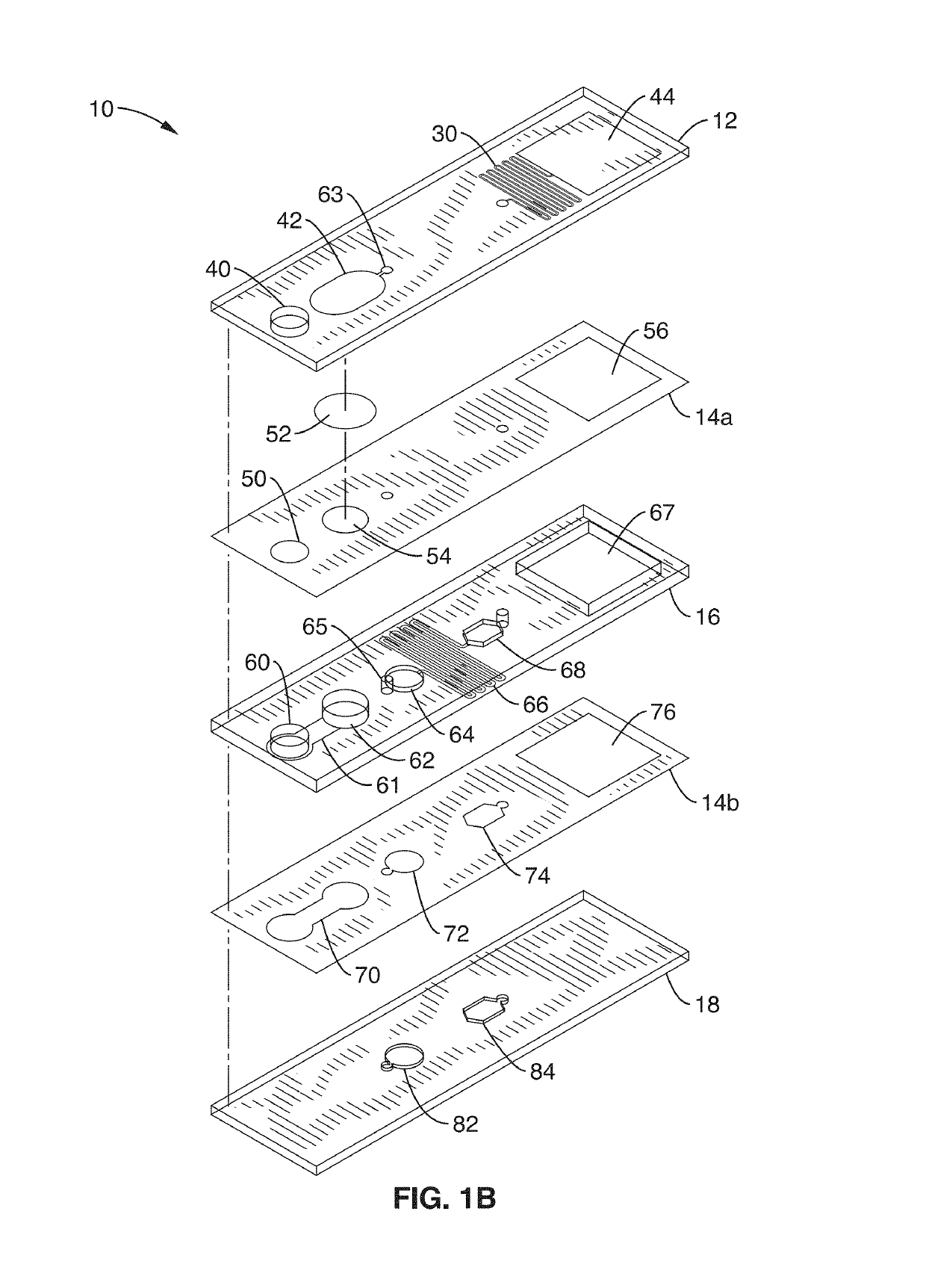
Integrated molecular diagnostics system (IMDX) and method for dengue fever
a molecular diagnostics and integrated technology, applied in the field of diagnostic systems and methods, can solve the problems of lack of sensitivity and/or specificity, and achieve the effect of improving 80% of methods and 80% of methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
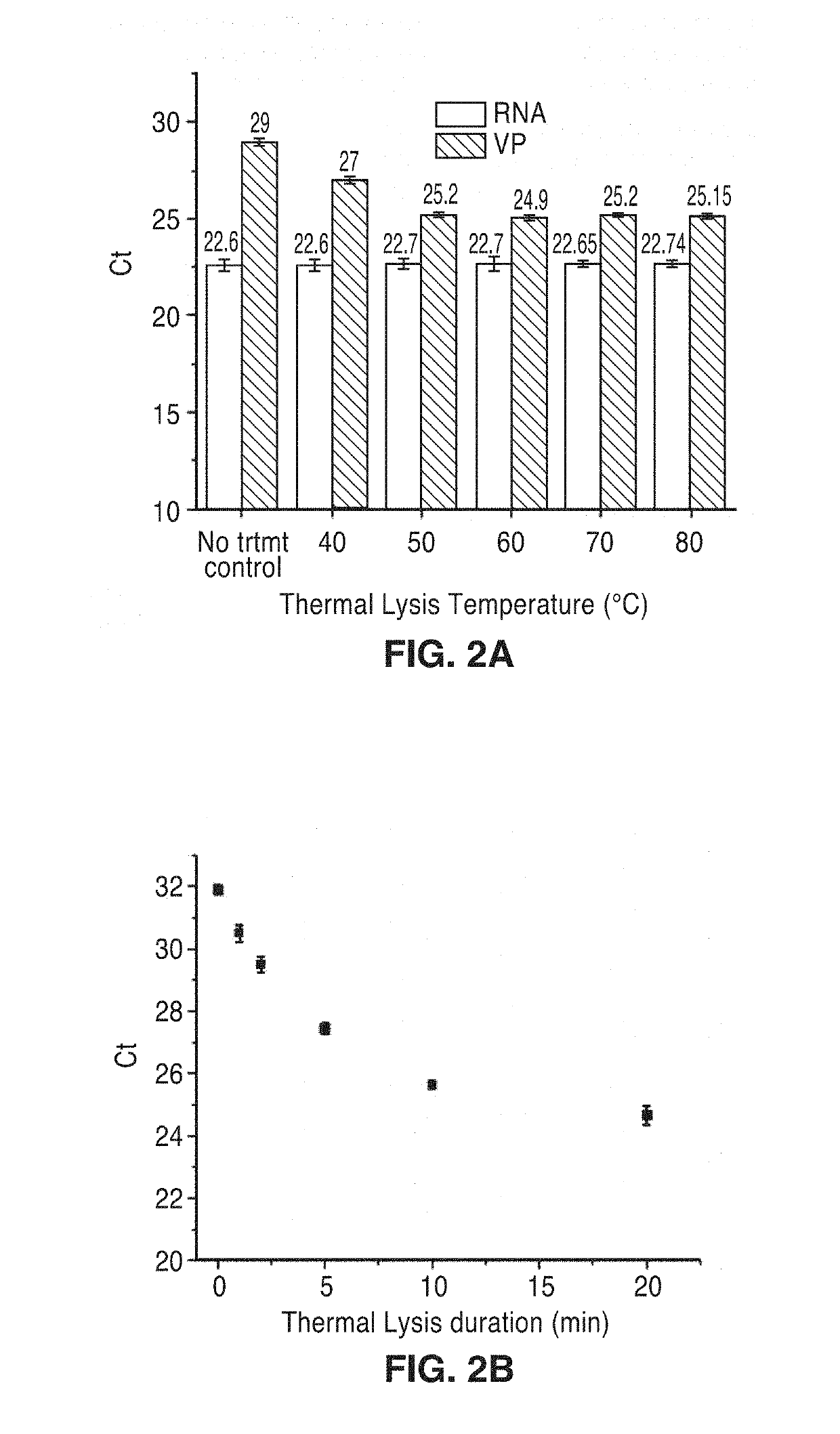
[0044]First, the temperature and time for the thermal lysis to extract the viral RNA from the Dengue virus were optimized as shown in FIG. 2A and FIG. 2B. As the temperature and time increase for the thermal lysis, the threshold values (Ct) are decreased at certain temperature (50° C.) and time (10 minutes), although the Ct for the thermal lysis are still higher than the extracted RNA. This could be attributed to the RNA damage caused by the formaldehyde inactivation of Dengue virus. The effects of the percentages of the blood plasma during RT-qPCR and thermal lysis were investigated as shown in FIG. 2C and FIG. 2D. With 20% of blood plasma, the RT-qPCR was significantly inhibited, showing high threshold cycle, although the RNAse inhibitor was included during the reaction. Also, as the percentages of the plasma increase during thermal lysis, the threshold value is increased. Therefore, to obtain single step Dengue Fever on-chip detection without compromising the sensitivity, the max...
example 2
[0046]To investigate the selective Dengue virus separation from the whole blood, the whole blood plasmas containing different concentrations of the Dengue virus were separated using the conventional off-chip centrifugation and on-chip blood plasma separation device. After blood plasma separation, the viral RNA was extracted and RT-qPCR was performed. FIG. 4A shows the real-time fluorescent intensities for the RT-qPCR of Dengue virus. The RT-qPCR with blood plasma separated by on-chip method shows similar threshold cycles with blood plasma prepared by conventional centrifugation method as shown in FIG. 4B. FIG. 4C shows the 2% agarose gel result confirming the formation of the specific PCR products during RT-qPCR with blood plasma containing different concentrations of the Dengue virus. The viral particles recovery can be further improved by optimizing the surface passivation of the plastic surface of the iMDx 10.
example 3
[0047]An on-chip LED-driven photothermal lysis in optical cavity chamber with two thin gold films (10 nm and 120 nm thicknesses) was also performed as shown in FIG. 5. The blood plasmas containing different concentrations of the Dengue virus were thermally treated to extract the viral RNA. For the comparison purpose, the Bio-Rad PCR thermal cycler was used for the off-chip thermal lysis. As shown in FIG. 5B and FIG. 5C, the on-chip LED-driven photothermal lysis shows the similar results with the off-chip thermal lysis, indicating that this on-chip thermal lysis can effectively extract the viral RNA from Dengue virus for the sample-to-answer molecular diagnostics device at point-of-care.
[0048]Finally, the LED-driven rapid optical cavity PCR was employed to amplify the Dengue cDNA as shown in FIG. 6. The optical cavity with 10 nm and 120 nm thicknesses was used for the PCR. The amplification of Dengue cDNA template was successfully demonstrated with concentrations ranging from 103 to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com