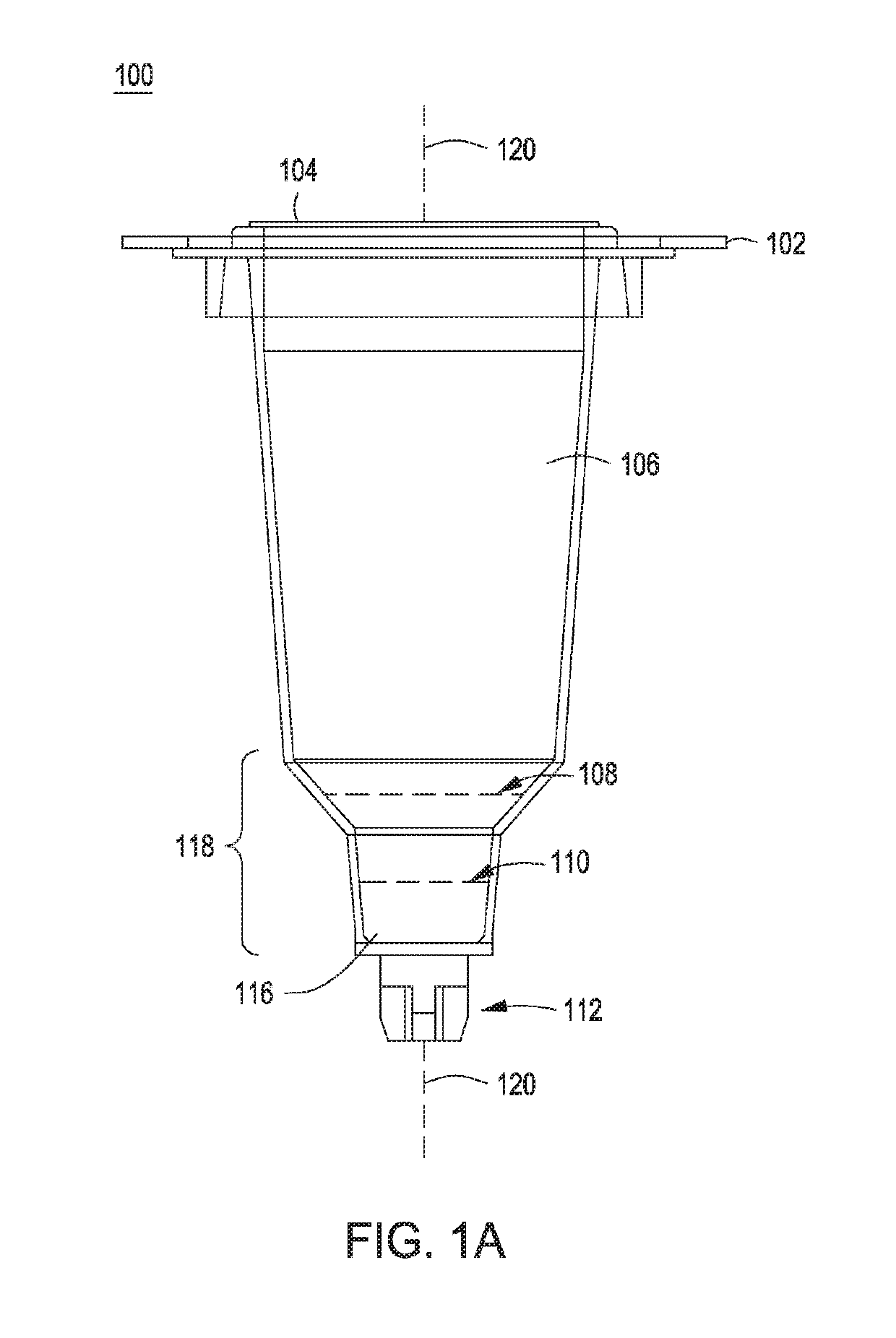
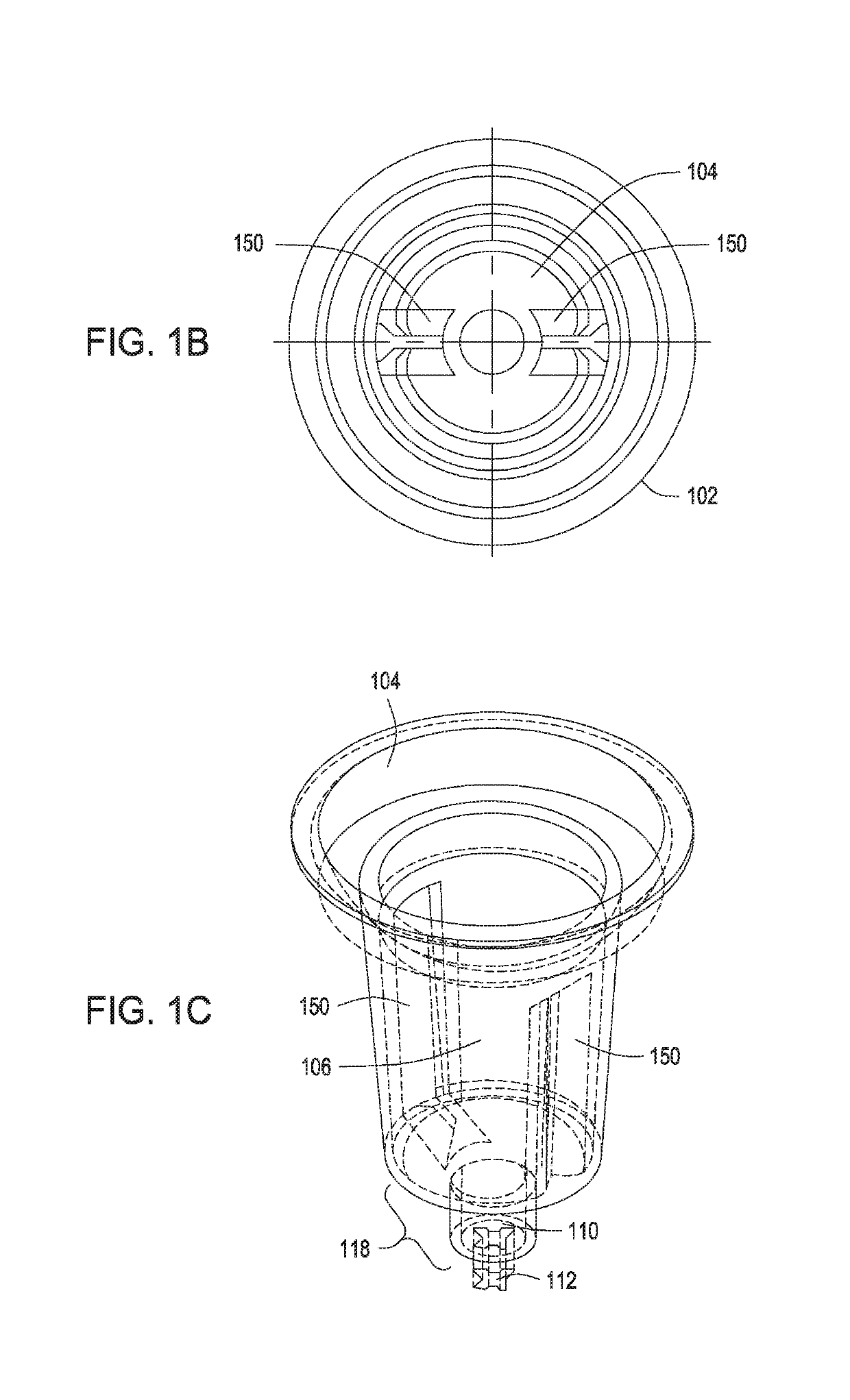
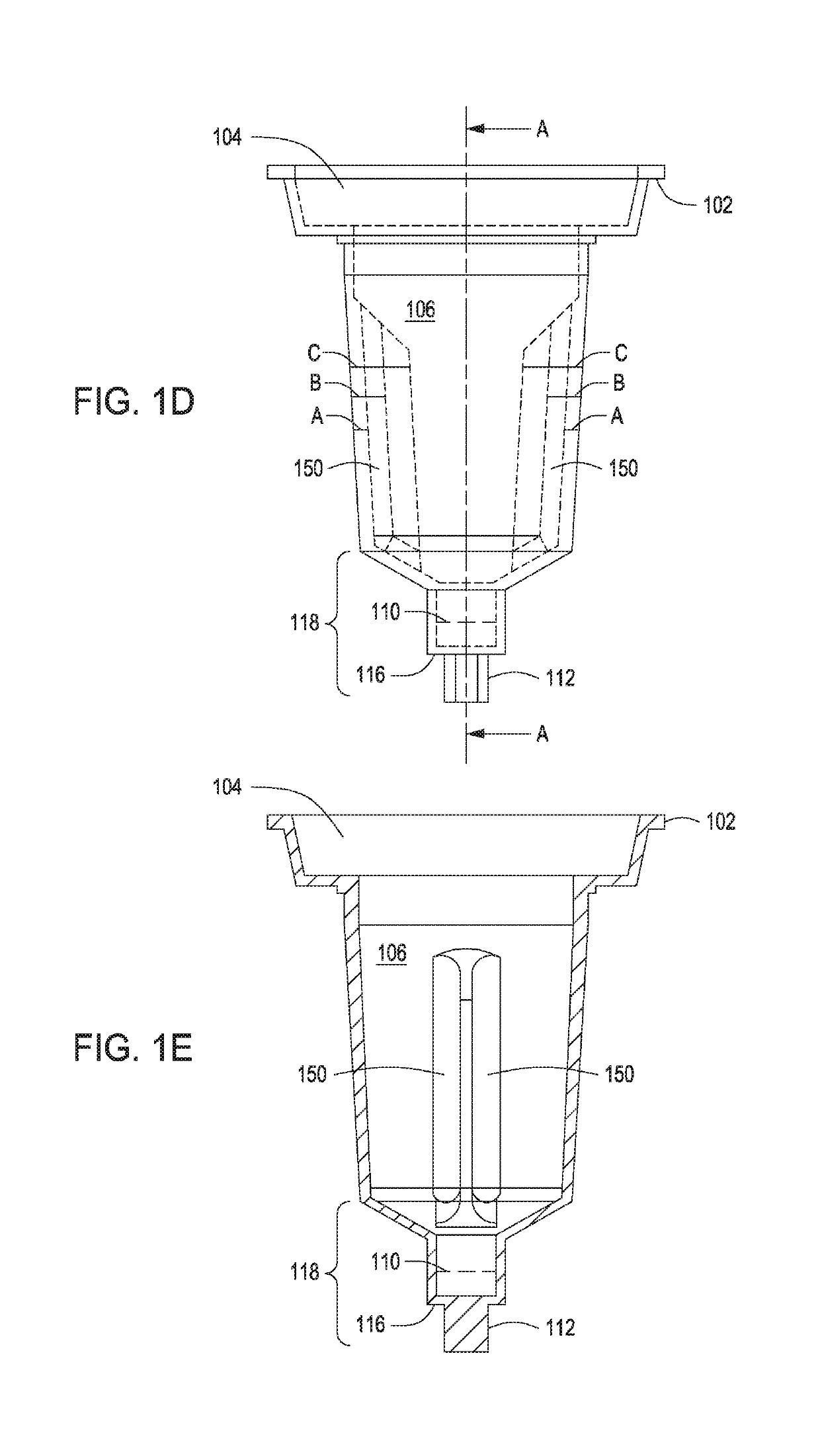
Automated control of cell growth rates for induction and transformation
a cell growth rate and induction technology, applied in biochemical apparatus and processes, specific use bioreactors/fermenters, after-treatment of biomass, etc., can solve the problems of contaminated cell culture, time and effort required for procedures, and human intervention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Growth in the Cell Growth Module
[0113]One embodiment of the cell growth device as described herein was tested against a conventional cell shaker shaking a 5 ml tube and an orbital shaker shaking a 125 ml baffled flask to evaluate cell growth in bacterial and yeast cells. Additionally, growth of a bacterial cell culture and a yeast cell culture was monitored in real time using an embodiment of the cell growth device described herein.
[0114]In a first example, 20 ml EC23 cells (E. coli cells) in LB were grown in a 35 ml rotating growth vial with a 2-paddle configuration at 30° C. using the cell growth device as described herein. The rotating growth vial was spun at 600 rpm and oscillated (i.e., the rotation direction was changed) every 1 second. In parallel, 5 ml EC23 cells in LB were grown in a 5 ml tube at 30° C. and were shaken at 750 rpm. OD600 was measured at intervals using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific). The results are shown in FIG. 8. The rotating gro...
example ii
Fully-Automated Singleplex RGN-directed Editing Run
[0118]Singleplex automated genomic editing using MAD7 nuclease was successfully performed with an automated multi-module instrument of the disclosure. See U.S. Pat. No. 9,982,279.
[0119]An ampR plasmid backbone and a lacZ_F172* editing cassette were assembled via Gibson Assembly® into an “editing vector” in an isothermal nucleic acid assembly module included in the automated instrument. lacZ_F172 functionally knocks out the lacZ gene. “lacZ_F172*” indicates that the edit happens at the 172nd residue in the lacZ amino acid sequence. Following assembly, the product was de-salted in the isothermal nucleic acid assembly module using AMPure beads, washed with 80% ethanol, and eluted in buffer. The assembled editing vector and recombineering-ready, electrocompetent E. Coli cells were transferred into a transformation module for electroporation. The cells and nucleic acids were combined and allowed to mix for 1 minute, and electroporation w...
example iii
Fully-Automated Recursive Editing Run
[0122]Recursive editing was successfully achieved using the automated multi-module cell processing system. An ampR plasmid backbone and a lacZ_V10* editing cassette were assembled via Gibson Assembly® into an “editing vector” in an isothermal nucleic acid assembly module included in the automated system. Similar to the lacZ_F172 edit, the lacZ_V10 edit functionally knocks out the lacZ gene. “lacZ_V10” indicates that the edit happens at amino acid position 10 in the lacZ amino acid sequence. Following assembly, the product was de-salted in the isothermal nucleic acid assembly module using AMPure beads, washed with 80% ethanol, and eluted in buffer. The first assembled editing vector and the recombineering-ready electrocompetent E. Coli cells were transferred into a transformation module for electroporation. The cells and nucleic acids were combined and allowed to mix for 1 minute, and electroporation was performed for 30 seconds. The parameters fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com