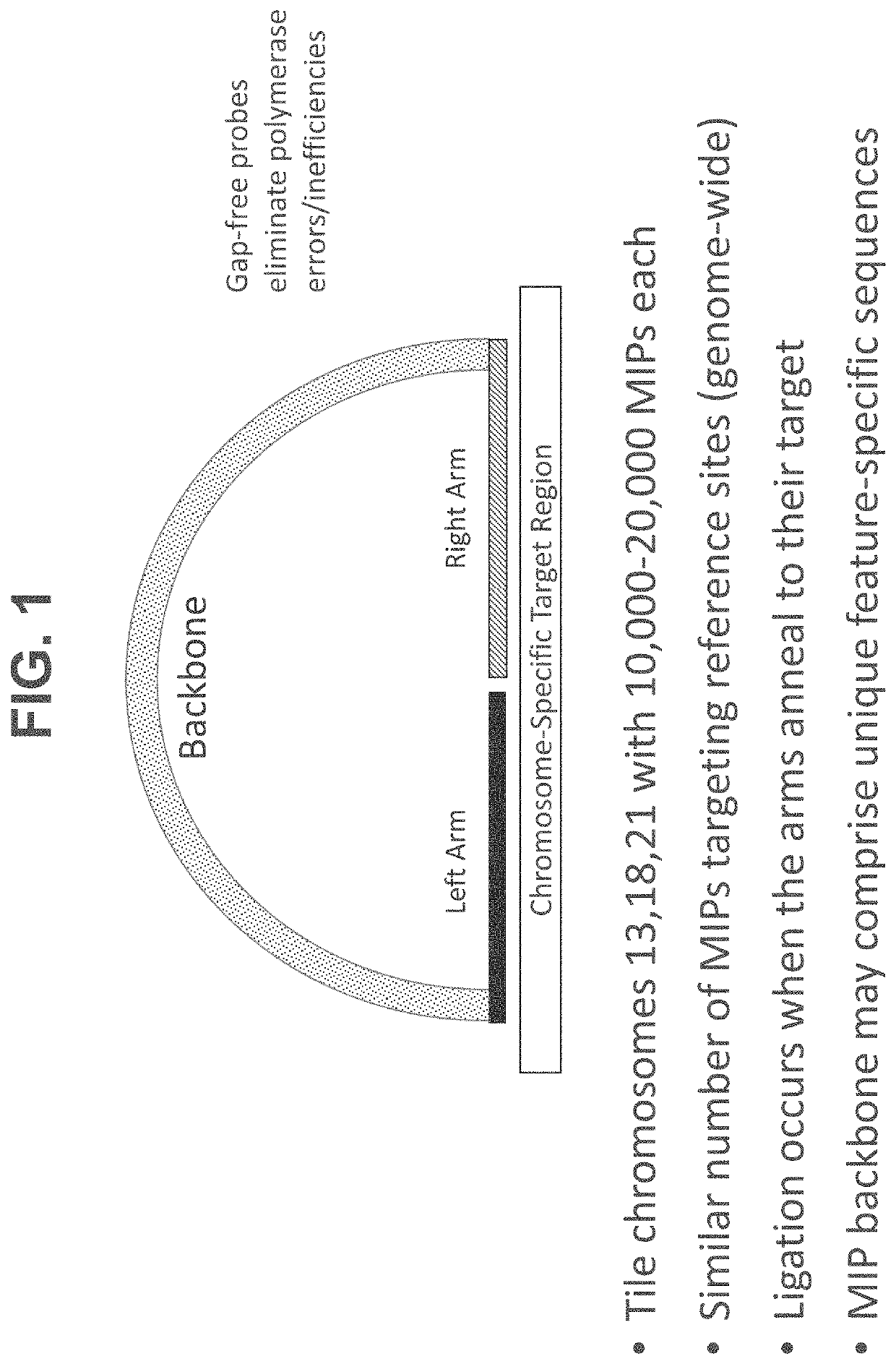
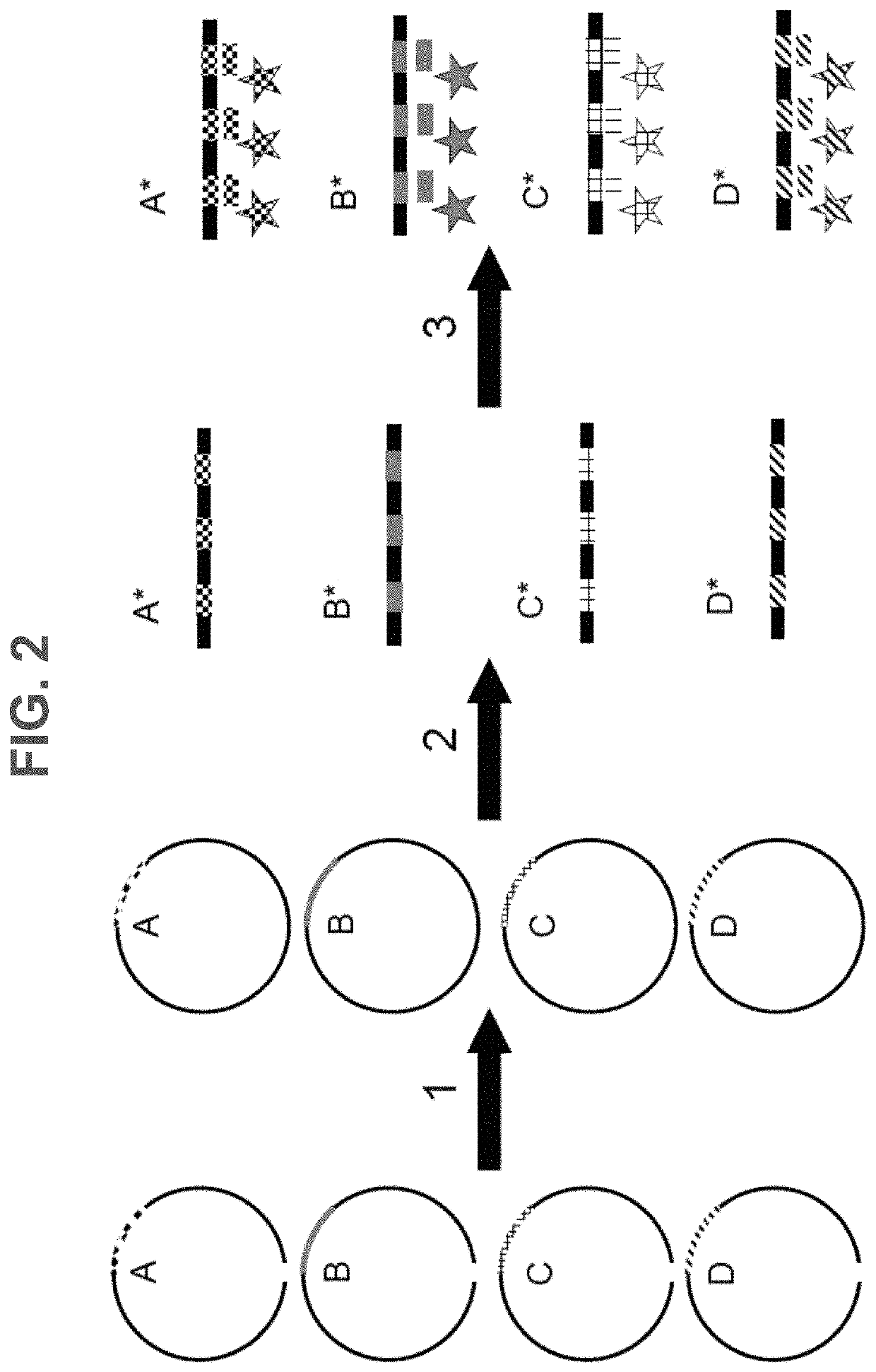
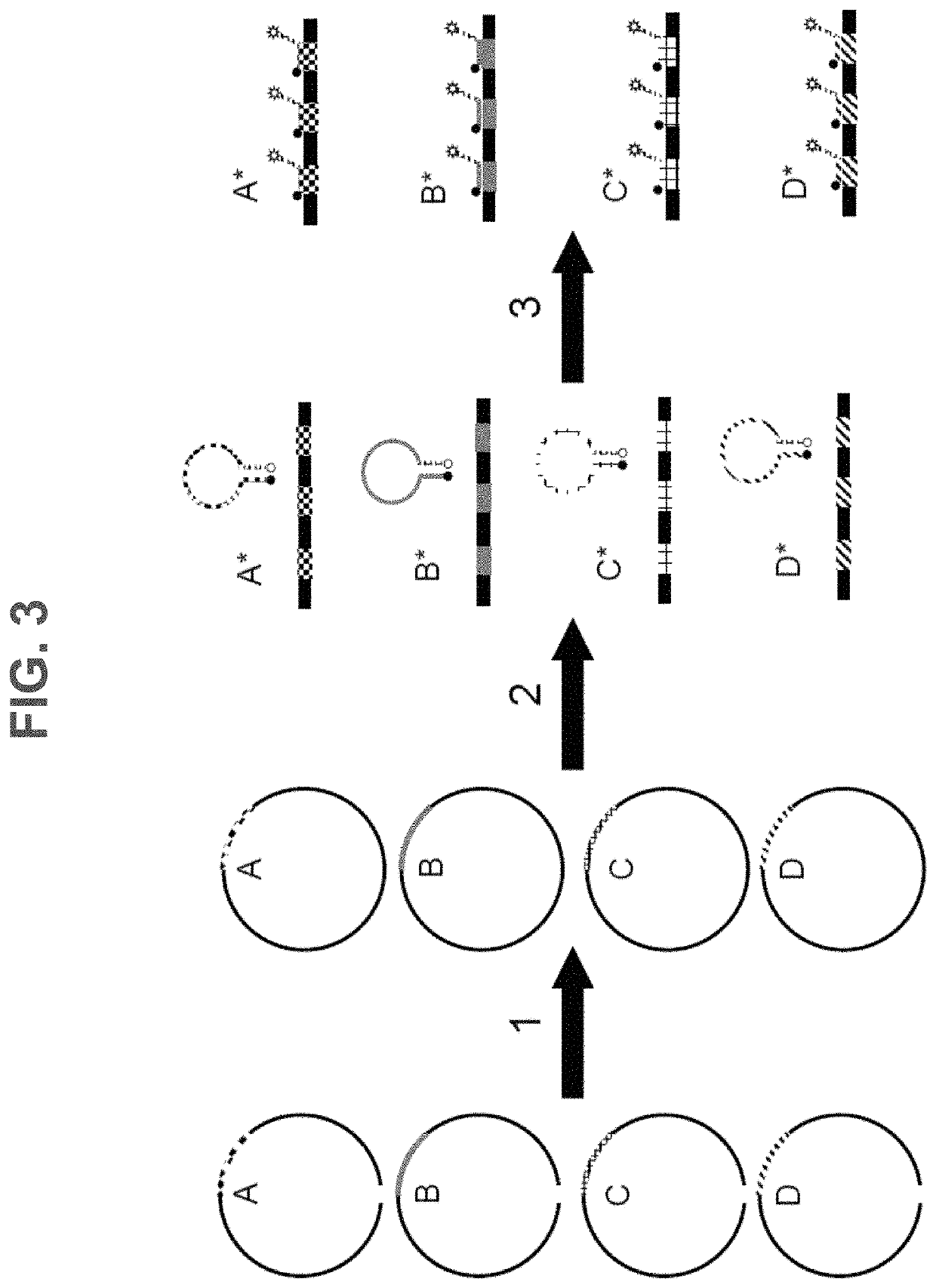
Methods, systems, and compositions for counting nucleic acid molecules
a technology of nucleic acid molecules and compositions, applied in the field of methods, systems and compositions for counting nucleic acid molecules, can solve the problems of fetal aneuploidy, inability to fully realize the effect of fetal aneuploidy, and inability to fully realize the effect of fetal aneuploidy, and achieve the effect of reducing the risk of fetal aneuploidy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0218]This example provides examples of work-flows for analysis of DNA, e.g., cfDNA, from a sample such as a blood sample.
[0219]Blood is collected in a standard draw from patient. A 10 mL of blood stored in a Streck blood collection tube or alternative EDTA-containing blood collection tube. The sample is transported into a lab at ambient temperature and processed as follows:[0220]Centrifuge blood at 2000×g for 20 minutes at room temperature to obtain a plasma fraction from the blood.[0221]Transfer plasma into a new, sterile, nuclease-free polypropylene tube and centrifuge at 3220×g for 30 minutes.
Cell-Free DNA (cfDNA) Purification
[0222]Cell-free DNA is purified from plasma using standard methods, e.g., using a MagMAX Cell-Free DNA isolation kit (Thermofisher Scientific, Cat. No. A29319).
Assay Plate Preparation
[0223]Glass bottom microtiter plates are treated to immobilize an oligonucleotide that primes the rolling circle amplification of a circularized MIPs. Several ...
example 2
Detection Using Two-Step Rolling Circle Amplification on a Surface with Graphene Oxide
[0322]Prepare Rolling Circle Amplification (RCA) solution on ice:[0323]For a 100 μL RCA solution (without molecular beacon), combine:[0324]MIP probe-target DNA preparation (e.g., entire MIP capture / cfDNA preparation described above, approximately 20 μL);[0325]10 μL of 10×Phi29 Buffer for a 1× final concentration[[0326]1×Phi29 DNA Polymerase Reaction Buffer[0327]50 mM Tris-HCl[0328]10 mM MgCl2 [0329]10 mM (NH4)2SO4 [0330]4 mM DTT[0331](pH 7.5 @ 25° C.)[0332]4 μL of 10 mM dNTPs, for a 0.4 mM total dNTPs final concentration;[0333]50 μL of filtered 30% PEG 600;[0334]8 μL of Phi29 polymerase (10 units / μL); and[0335]23 μL of molecular-grade water[0336]Mix solution by vortexing and pipet onto treated glass surface, then seal plate;[0337]Incubate plate on flat bottom heat block of thermomixer with a thermo-lid, at 45° C. for 90 minutes;[0338]Remove well contents and wash well three times with 100 μL of 1×T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting temperature | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com