Multiplexed immunosignal amplification using hybridization chain reaction-based method
a multi-assay, hybridization chain technology, applied in the field of antibody-based immunoassays, can solve the problems of reducing spatial resolution, difficult to use for simultaneous detection of multiple amplified signals, unsuitable for large-volume samples in several powerful new tissue expansion and clearing techniques, etc., to achieve substantial fluorescence recovery, suppress background levels, and eliminate the fluorescence of hcr amplifiers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0051]Multiplexed Labeling Using isHCR.
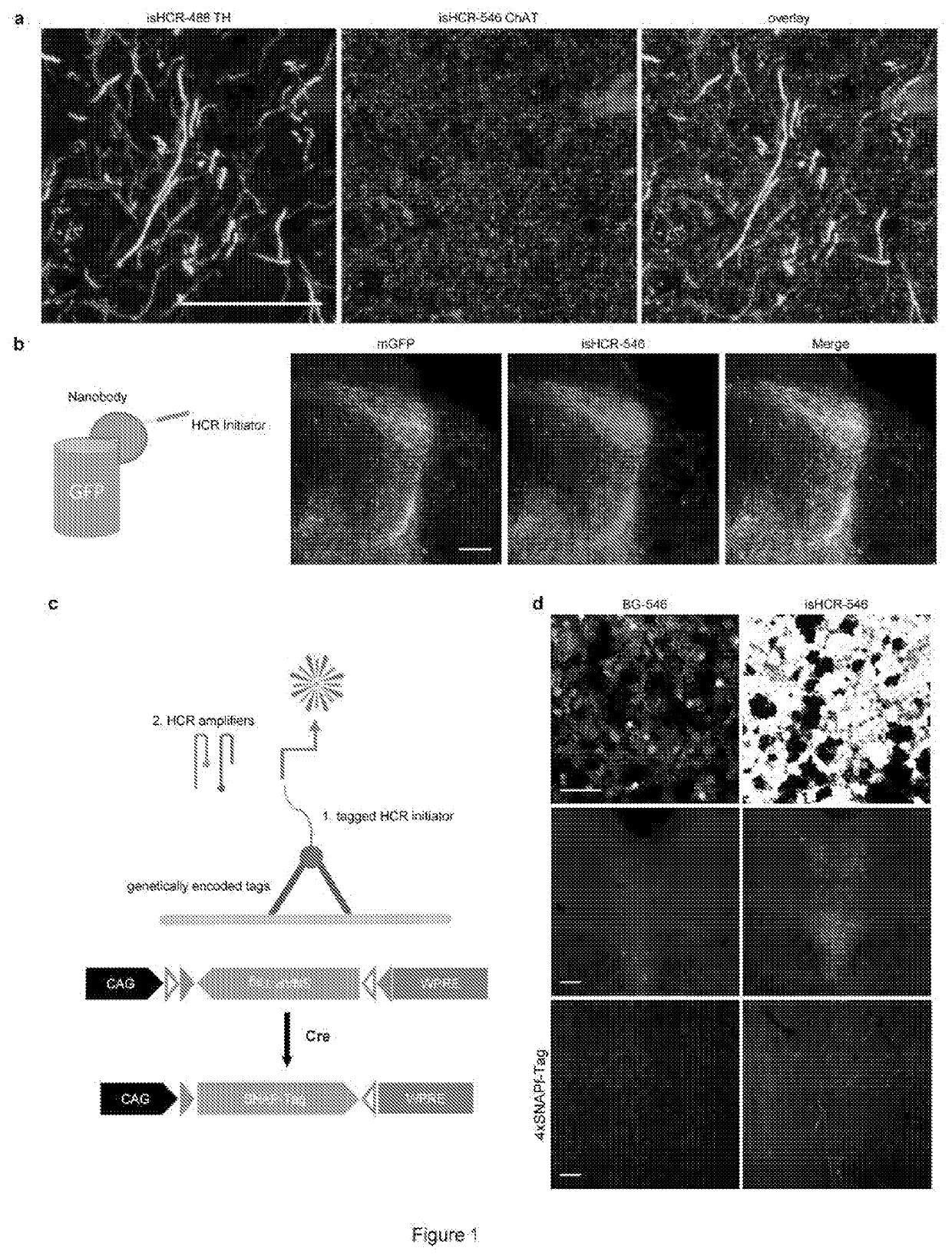
[0052]FIG. 1. (a) shows images of the dorsal striatum in mouse brain sections double immunostained for TH (green) and choline acetyltransferase (ChAT, red). The signals of each antigen were visualized sequentially using corresponding biotinylated secondary antibodies and isHCR. (b) HCR initiators were conjugated to GFP-nanobodies (LaG-16-2) using SMCC as the linker. mGFP proteins were expressed in the orbitofrontal cortex neurons in CaMKII-Cre transgenic mice using adeno-associated virus (AAV) vectors. The GFP signals in the superior colliculus were amplified using HCR initiator-conjugated GFP-nanobody and isHCR-546. (c) Schematic of rapid labeling using genetically encoded protein tags. Functionalized HCR initiators bind directly to protein tags and initiate the amplification process. AAV vectors that bear the Cre-dependent double-floxed inverse (DIO) open reading frame cassette containing genes encoding the SNAPf tag were constructed and pack...
example 2
[0053]Simultaneous Detection of Multiple Targets Using isHCR.
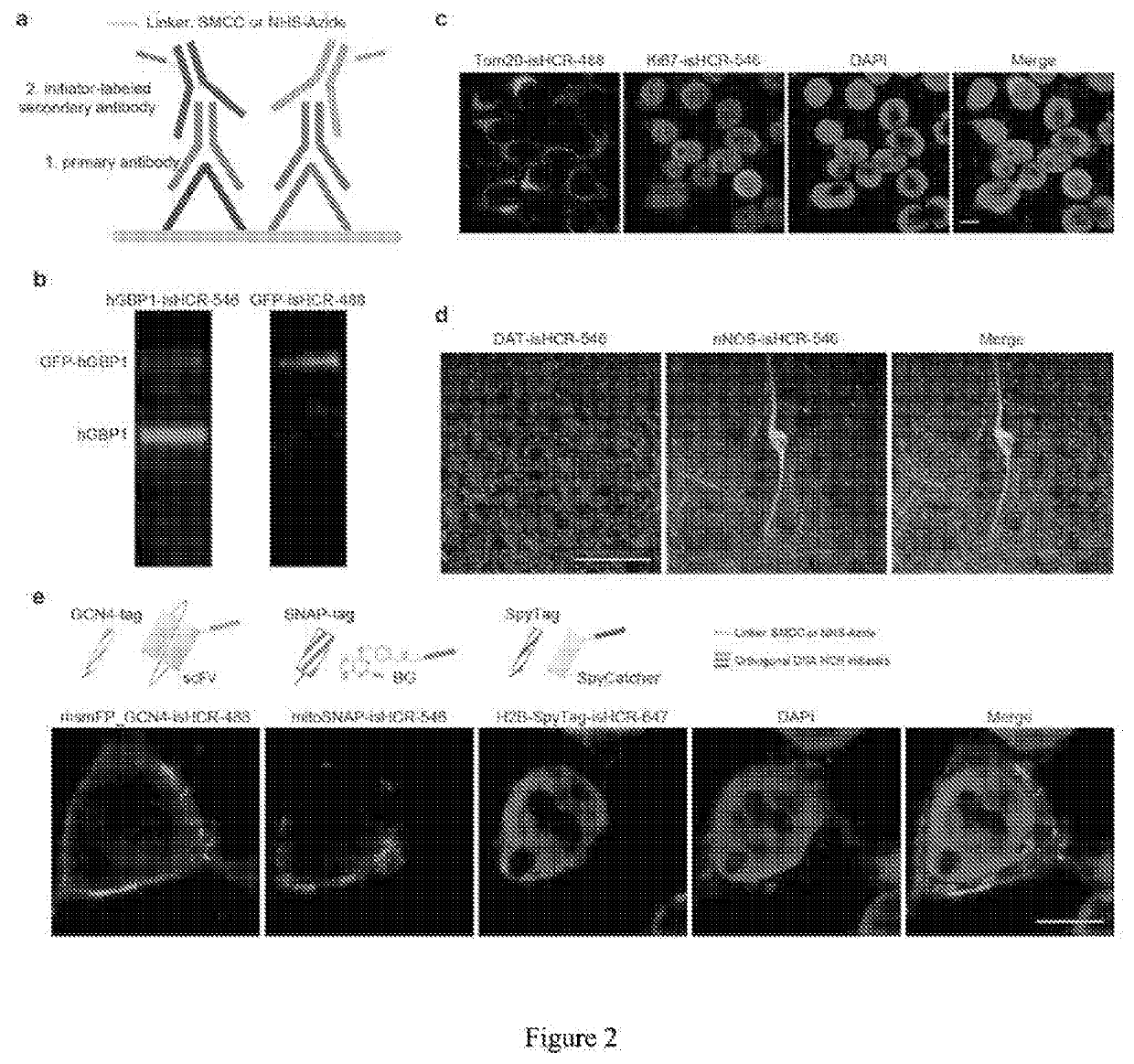
[0054]FIG. 2. (a) shows that two orthogonal HCR initiators can be conjugated directly onto secondary antibodies using either SMCC or Click Chemistry groups as linkers. (b) Western blot of a protein mixture containing purified hGBP1 and purified GFP-hGBP1 proteins. An anti-GFP primary antibody and an anti-hGBP1 primary antibody were applied. The two primary antibodies were detected using two secondary antibodies that were conjugated with different HCR initiators. (c) Images of HEK 293T cells immunostained against the nuclear protein Ki67 (red) and a mitochondria protein Tom20 (green) using two HCR initiator-conjugated secondary antibodies. The signals were then simultaneously amplified using isHCR-546 for Ki67 and isHCR-488 for Tom20. (d) Images of the dorsal striatum in mouse brain sections double immunostained against dopamine transporter (DAT, red) and neuronal nitric oxide synthase (nNOS, green) using two HCR initiator-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com