Formulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0262]The following formulation was prepared according to the method provided below where:
[0263]To prepare 9 Litres of Saline
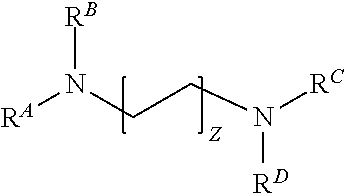
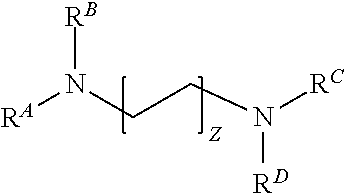
[0264]9 g—0.1 wt. % of propoxylated ethylenediamine compound according to Formula Ai having a number average molecular weight of 7200 (generally the compound sold under the trade name Tetronic® 90R4);
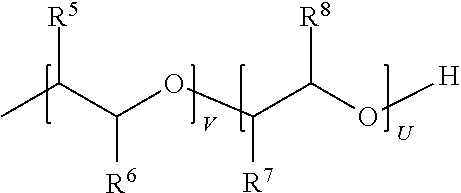
[0265]45 g—0.5 wt. % block copolymer of ethylene oxide and propylene oxide (PEG-PPG-PEG) having a number average molecular weight of 5800 (generally the compound sold under the trade name Pluronic® 123);
[0266]36 g—0.4 wt. % of Polyacrylic Acid (PAA) Sodium Salt having a number average molecular weight of 15000;
[0267]81 g—0.9 wt. % of Sodium Chloride;
[0268]9.9 g—0.1 wt. % of Boric Acid;
[0269]0.99 g—0.01 wt. % of Sodium di-Tertborate
[0270]0.45 g—0.005 wt % non-ionic, hydrophilic surfactant such as that sold under the trade name Poloxamer 407.
[0271]8910 mL of deionised water—98 wt %
[0272]1. Measure 946 ml of deionised water into the Duran bottle using a measuring cyl...
example 2
[0294]The following solutions were prepared:
[0295]Saline 6
[0296]To prepare 9 Litres of Saline B[0297]81 g of Sodium Chloride—0.89 wt %[0298]9.9 g of Boric Acid—0.11 wt %[0299]0.99 g of Sodium di-Tertborate—0.011 wt %[0300]0.45 g Poloxamer 407—0.005 wt %[0301]9000 mL of deionised water—99 wt %
[0302]Saline E
[0303]To prepare 9 Litres of Saline E[0304]9 g of Tetronic 90R4 (Mn 7200)—0.1 wt %[0305]45 g Pluronic 123 (Mn 5800)—0.5 wt %[0306]36 g of Polyacrylic acid Sodium Salt (Mw 15000)—0.4 wt %[0307]81 g of Sodium Chloride—0.9 wt %[0308]9.9 g of Boric Acid—0.1 wt %[0309]0.99 g of Sodium di-Tertborate—0.01 wt %[0310]0.45 g Poloxamer 407-0.005 wt %[0311]8910 mL of deionised water—98 wt %
[0312]Saline F
[0313]To prepare 9 Litres of Saline F[0314]9 g of Tetronic 1107 (Mn)—0.099 wt %[0315]45 g Pluronic 123 (Mn 5800)—0.49 wt %[0316]0.6750 g Hydroxpropylmethyl cellulose—0.0074 wt. %[0317]9 g polyvinylpyrrolidone—0.099 wt %[0318]54 g of Polyacrylic acid Sodium Salt (Mw 15000)—0.59 wt %[0319]75.95 g...
example 2a
[0330]Lenses were swollen in heptane for 1 h than transferred into Solution IV for 3 h and transferred and autoclaved in Saline F. The contact angle of the treated lenses was measured at room temperature. The mean contact angle of the treated lens was 18.2 with an associated standard deviation of 5.3.
TimeSampleSampleSampleSampleSampleSampleSampleSampleSampleSample(min)12345678910Untreated 82.379.954.382.579.190.981.184.381.265.4lens15 to 3013.413.368.728.714.620.416.215.122.514.5
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Aggregation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com