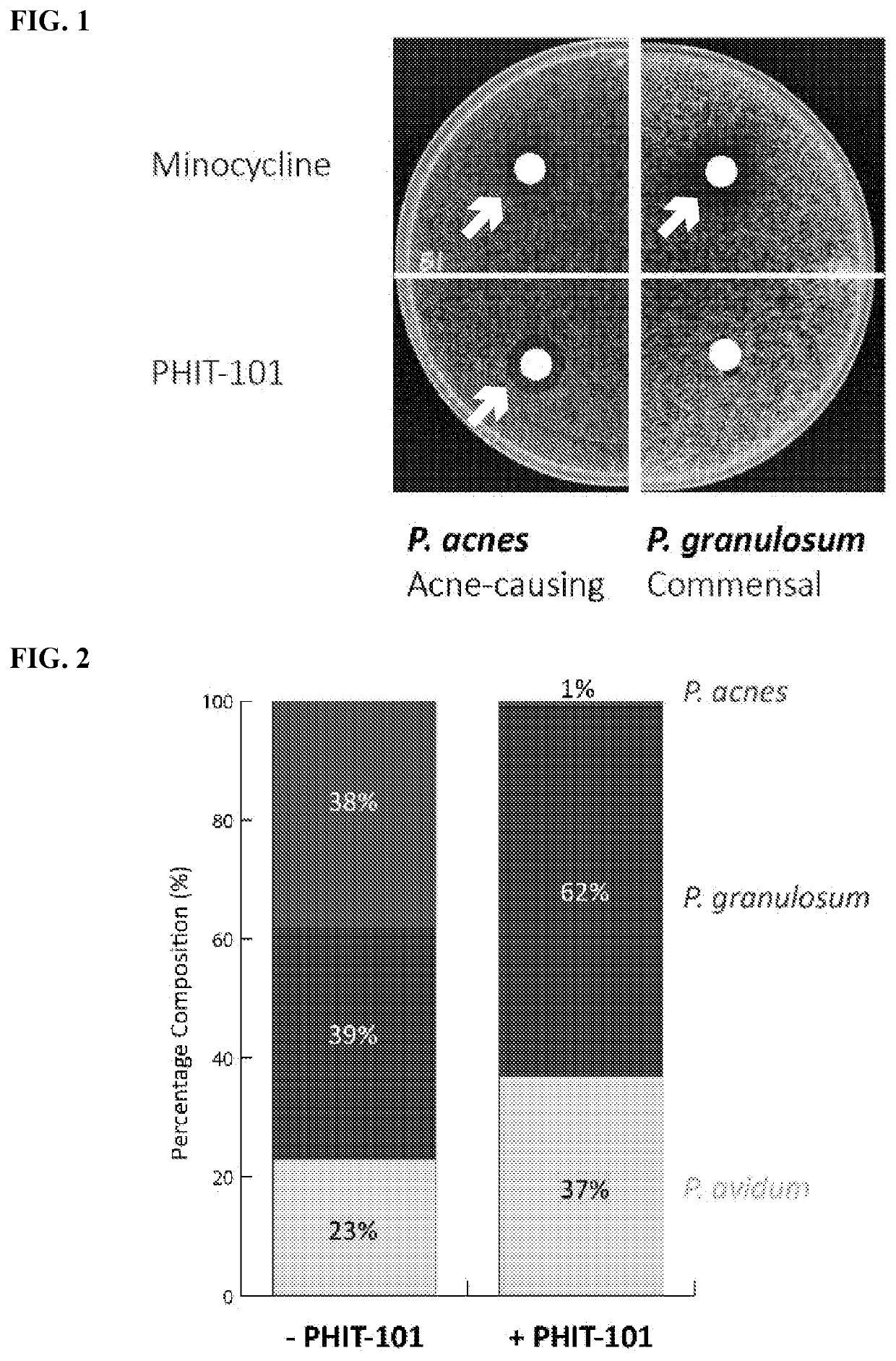
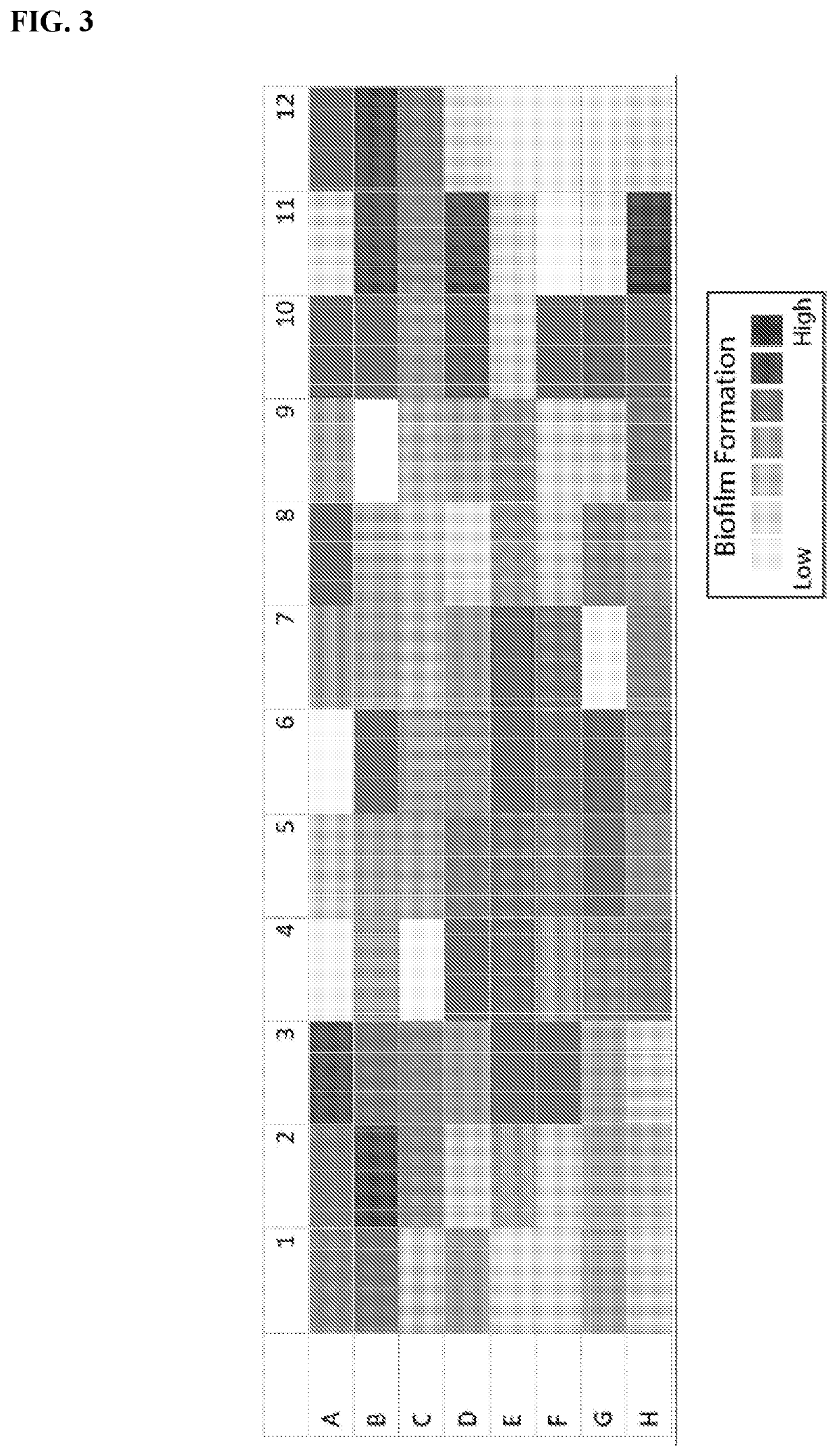
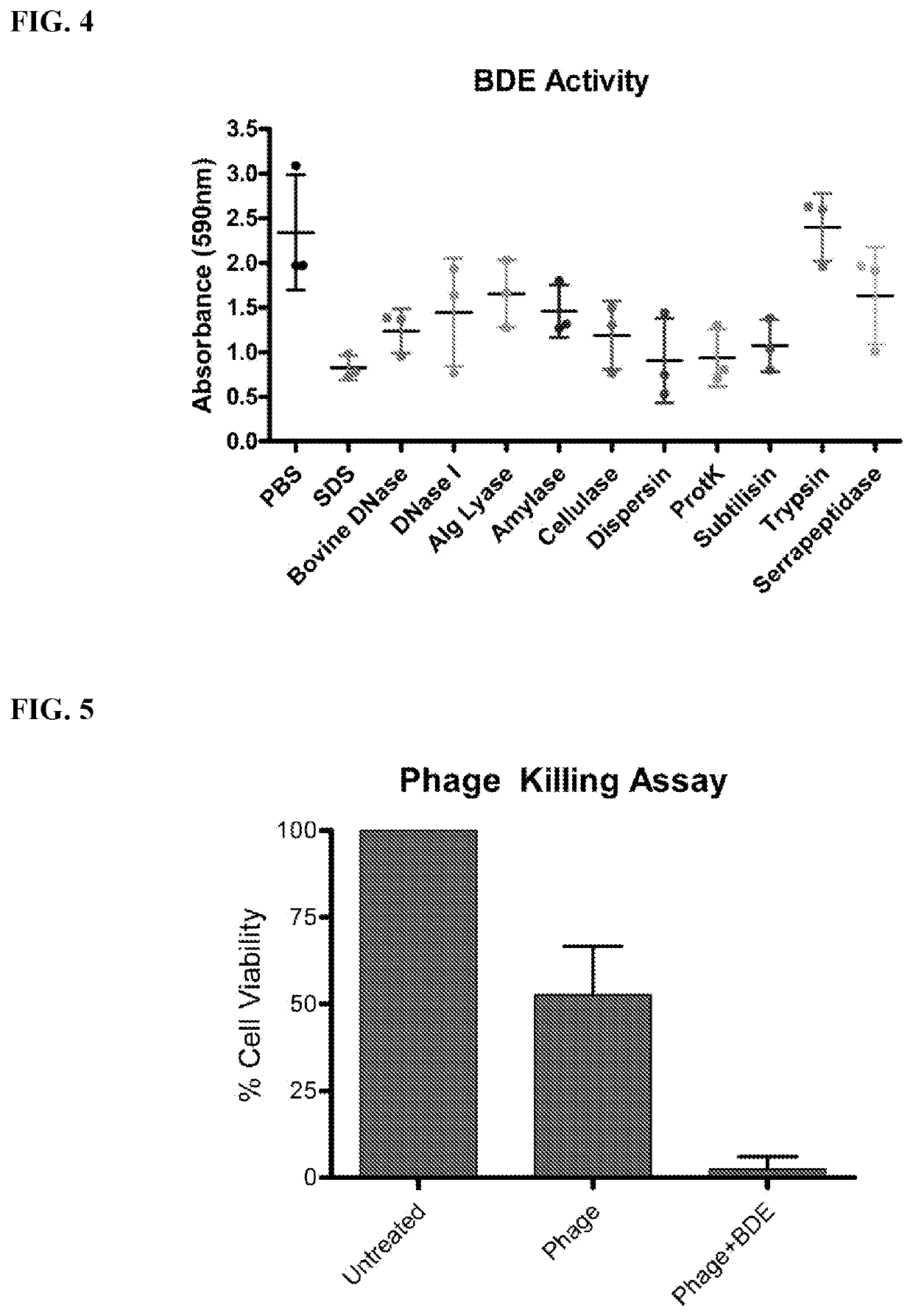
Compositions comprising propionibacterium acnes bacteriophages for treating acne
a technology of acne and bacteriophages, which is applied in the direction of viruses/bacteriophages, drug compositions, peptides/protein ingredients, etc., can solve the problems of not being able to develop a novel acne drug in over 30 years, current treatments including benzoyl peroxide and antibiotics are quite ineffective, and the most effective treatment—isotretinoin—is limited to a small set of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0184]Embodiments and examples are provided below to facilitate a more complete understanding of the invention. The following embodiments and examples illustrate the exemplary modes of making and practicing the invention. However, the scope of the invention is not limited to specific embodiments disclosed in these embodiments and examples, which are for purposes of illustration only, since alternative methods can be utilized to obtain similar results.
[0185]Embodiments include Embodiments P1 to P56 following:
[0186]Embodiment P1. A composition consisting essentially of at least one Propionibacterium acnes bacteriophage, at least one anti-acne compound, and a pharmaceutically acceptable carrier.
[0187]Embodiment P2. A composition comprising at least one Propionibacterium acnes bacteriophage, at least one anti-acne compound, and a pharmaceutically acceptable carrier, wherein the composition does not comprise a probiotic bacterium.
[0188]Embodiment P3. The composition of Embodiment P2, whe...
example 2
Genotypic Characterization of Probiotic Strains.
[0305]Strains of P. acnes were characterized based on point mutations in the 16S rDNA sequence which leads to phylogenetic sorting into pathogenic and probiotic strain types, and the absence of a linear plasmid found in pathogenic strains, which carries virulence factors. Using 16S-specific primers the full 16S rDNA sequence of each P. acnes strain was amplified and Sanger-sequenced. A probiotic strain was identified as having ribosequence (RS) of ProI or ProII. ProI strains have T838C and C1322T mutations relative to the KPA171202 type strain's 16S rDNA sequence (NIH Accession No. NC_006085.1). ProII strains have C986T and T992C mutations relative to the KPA171202 sequence. Further, using specific primer pairs, the presence or absence of a linear plasmid within each strain was determined. Probiotic strains were identified as lacking this plasmid, which carries an extrachromosomal lipase as well as the Tad (tight adhesion) viru...
example 3
hage Stability in Compositions with Anti-Acne Compounds
[0314]In order to determine whether the phage was stable in co-formulation with either salicylic acid or benzoyl peroxide (BPO), the phage was co-incubated with these agents at a low and high concentration. The range of concentrations was determined by the permitted concentrations of these agents specified in the United States Food and Drug Administration (FDA) acne monograph for over-the-counter use. For salicylic acid, this is 0.5% to 2% (w / v), while for BPO the range is 2.5% to 10% (w / v). Buffered solutions of phage were added to these agents, and its stability at 4° C. was tested over 60-90 days. FIG. 15 shows that the phages are stable in the presence of both low and high doses of salicylic acid. In contrast, FIG. 16 shows that benzoyl peroxide destabilizes the phages, and the observed rate of decrease in phage viability is steeper at a higher concentration of BPO.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| weight/volume | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com