Acellular red blood cell substitute
a technology of red blood cells and red blood cells, applied in the direction of immunoglobulins, peptides/protein ingredients, peptides/protein ingredients, etc., can solve the problems of reducing kidney performance, unable to carry sufficient oxygen to support life, and unable to meet the needs of life, and still producing substantial renal dysfunction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Stroma-Free Tetrameric Hemoglobin Solution
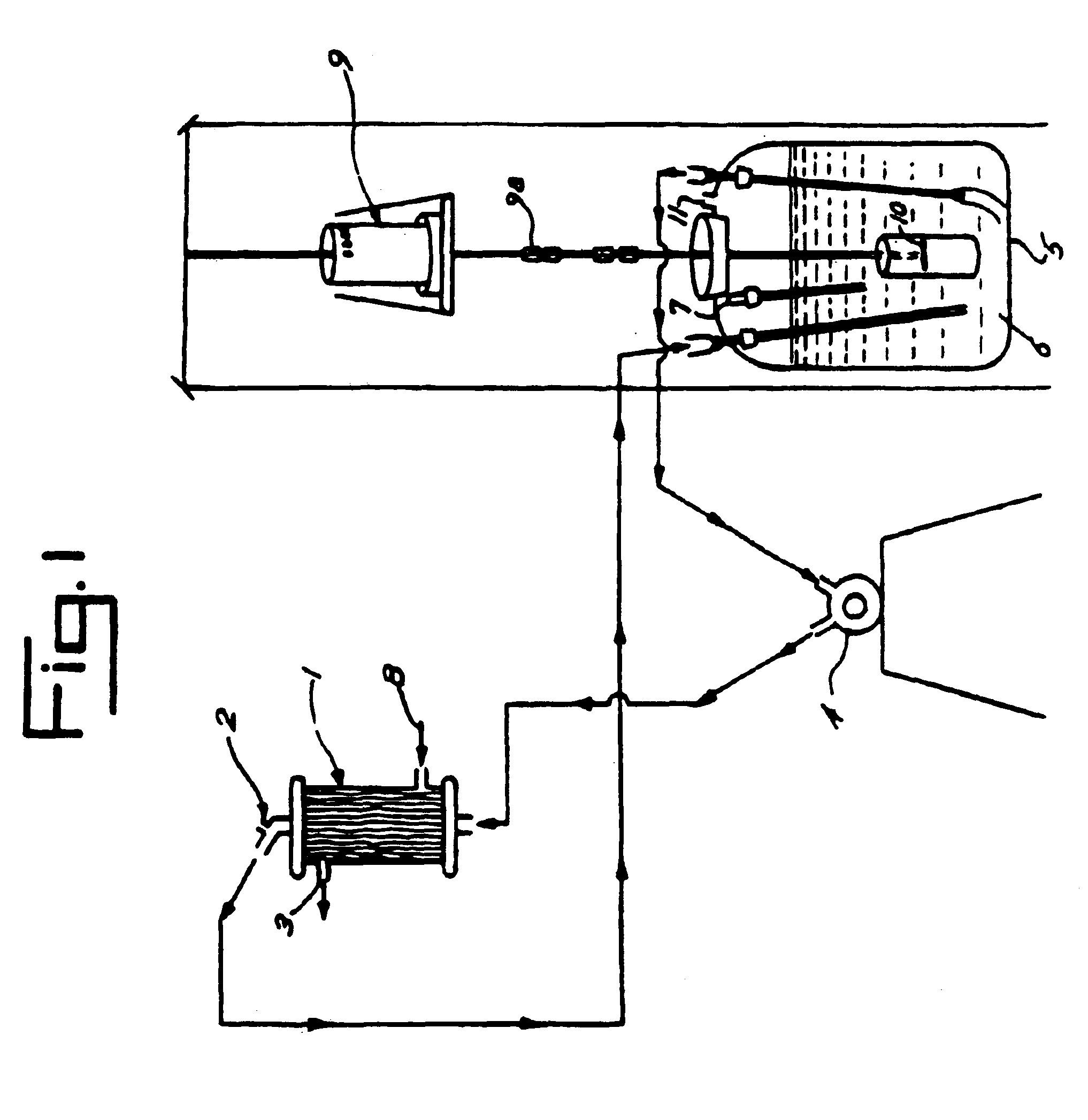
[0052]Outdated human blood was washed twice with 0.9% sodium chloride solution containing the following antibiotics per liter of solution:
[0053]
Penicillin50,000UStreptomycin50mgGentamycin40mgPolymixin B Sulfate2.5mg
[0054]The red blood cells (RBC) were washed twice with equal volumes of the above solution and centrifuged at 2,500 g for 15 minutes. Better than 95% of the buffy coat layer was removed with a plasma extractor (Fenwal Laboratories, Morton Grove, Ill.). The washed and packed RBCs were then pooled and lysed with 3 to 4 volumes of pyrogen free water. One hundred units of washed RBCs resulted in a 15-20 L volume at a hematocrit of 20-24%.
[0055]Fifteen to twenty liters of washed RBCs were poured into 40-80 liters of cold pyrogen free water. The lysate thus made, was then pumped through 2 to 4 0.1μ hollow fiber cartridges. An air driven pump was used (LP30, Amicon Corp., Danvers, Mass.). The 0.1μ cut-off cartridges were c...
example 2
Preparation of Stroma-Free Tetrameric Hemoglobin Solution—an Alternative Method
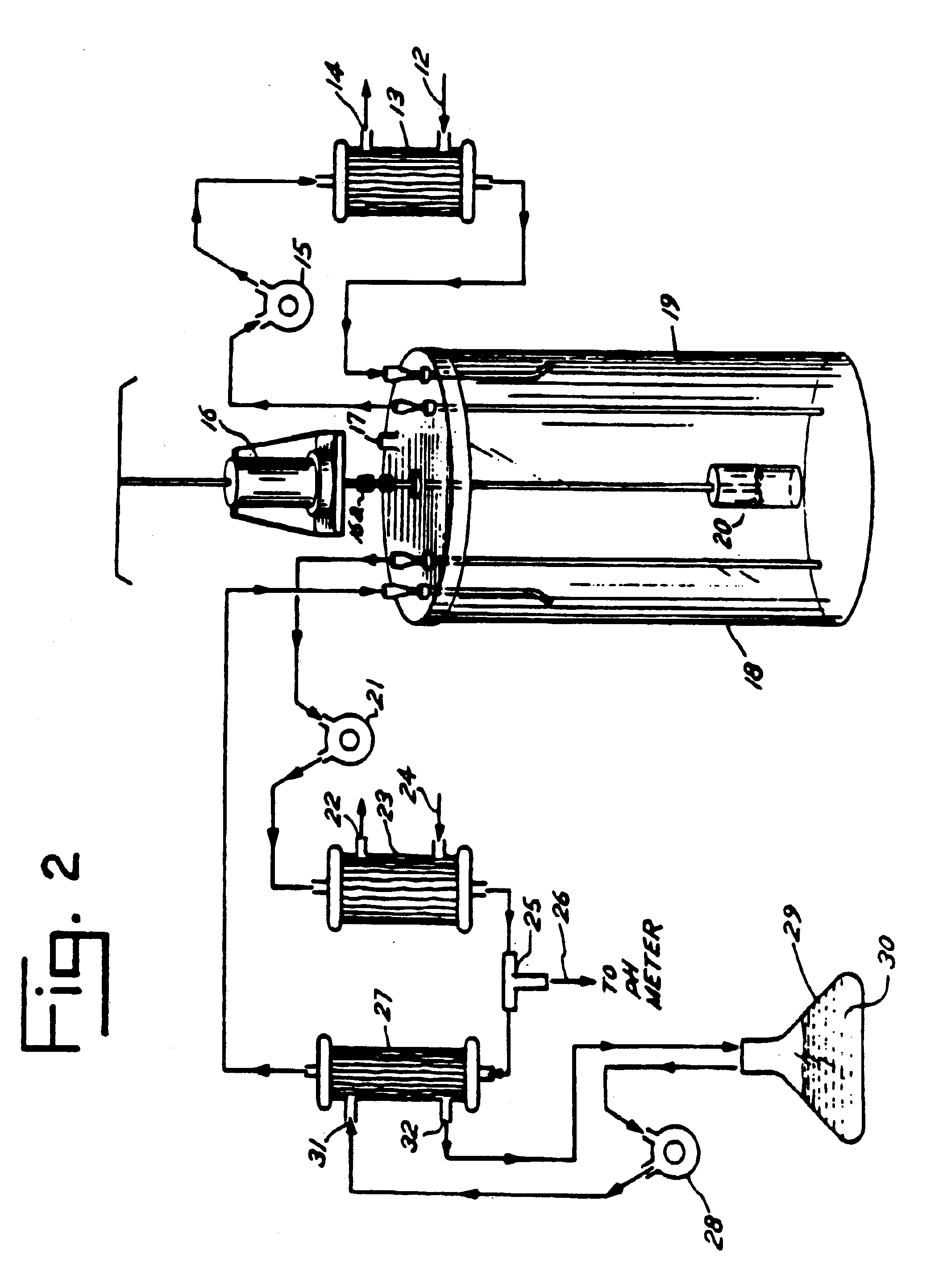
[0057]Individual units of outdated blood were filled with 0.9% sodium chloride solution. The red cells and buffy coat were allowed to settle overnight. The supernatant and buffy coat were then extracted.
[0058]The packed cells were then poured into 3-5 volumes of 0.9% sodium chloride solution. The cells were washed and concentrated by use of a 0.2μ hollow fiber cross-flow filter (K205—KROSFLOW, Microgon Corp., Laguna Beach, Calif.) and an air driven pump (LP30, Amicon Corp., Danvers, Mass.). The packed cells were then lysed by the addition of 3-5 volumes of pyrogen free water. The cross-flow filtration was then resumed, with the tetrameric hemoglobin along with the enzymic contents of the red cells being collected in the ultrafiltrate, while the stroma was retained by the filter.
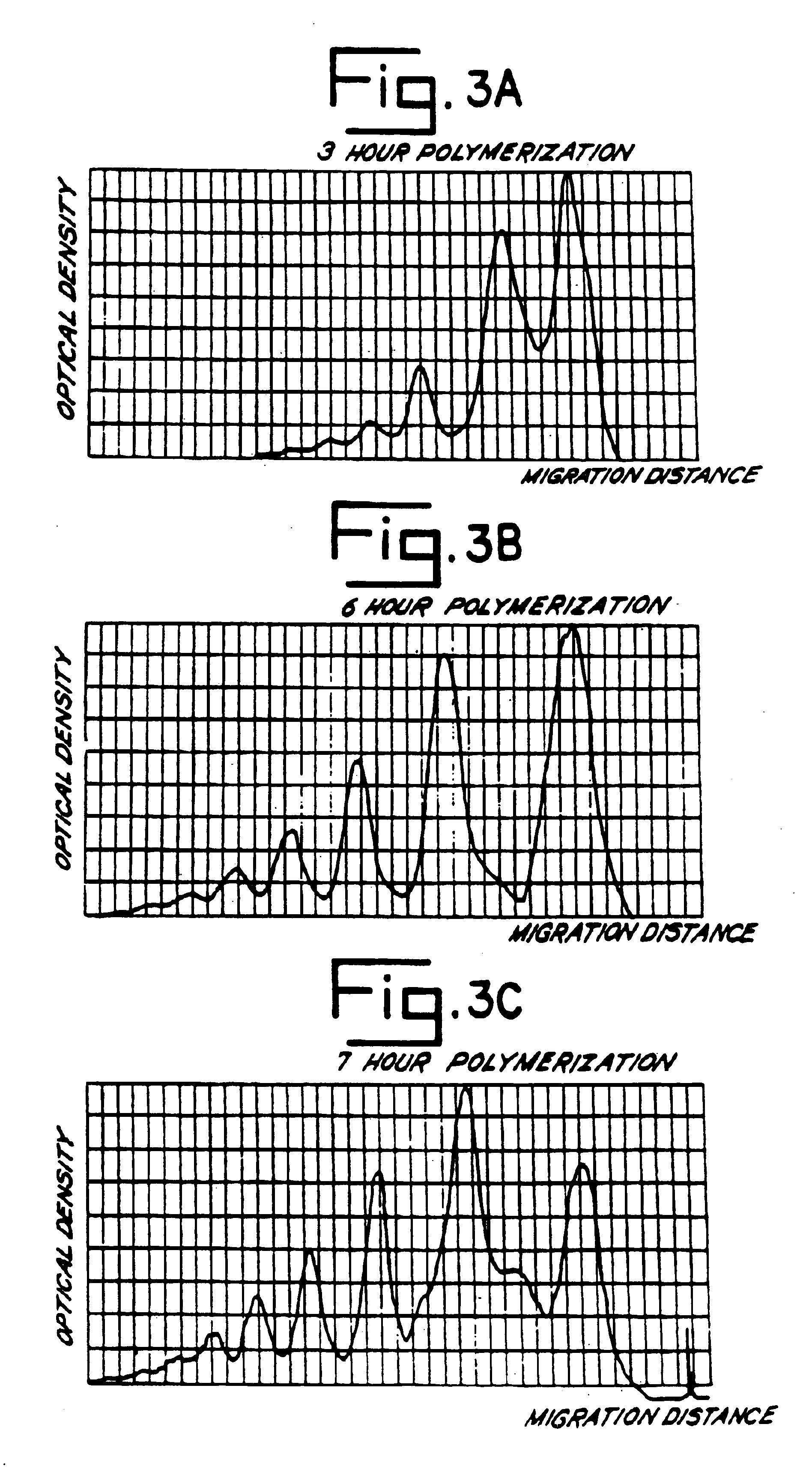
[0059]Aliquots of the filtrate were centrifuged several times during this process. Absence of a pellet reflected good membrane in...
example 3
Pyridoxylation of the Stroma-Free Hemoglobin—Mixed Batch Geometry
[0060]A stroma-free hemoglobin solution was prepared according to the process of Example 1 or 2. The following reagents were mixed together:[0061]1. Pyridoxal, 5′, phosphate—on a 4:1 molar ratio to hemoglobin;[0062]2. Tris-HCl buffer—0.1M final concentration in the hemoglobin solution;[0063]3. Glutathione—1 gm / L of solution;[0064]4. Ascorbic Acid—0.2 gm / L;[0065]5. Glucose 0.5 gm / L; and[0066]6. Antibiotics—the same antibiotics as described in Example 1.
[0067]The above reagents were dissolved in minimal volume of pyrogen free water and the pH adjusted to 7.25-7.45. The above mixture was added to the hemoglobin solution. The pH of the hemoglobin solution was adjusted to 7.35-7.45 at 5° C. with 0.1 N NaOH. Finally, the hemoglobin concentration was adjusted to 17.5-18.5 gm / dl.
[0068]The solution (18-20 L) was then transferred to a gas tight stainless steel reservoir. Deoxygenation of the solution was accomplished by use of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com