Self-renewing pluripotent hepatic stem cells
a hepatic stem cell and self-renewing technology, applied in the field of stem cells, can solve the problems of non-transformed hepatic stem cells and their progeny, and achieve the effect of reducing the number of stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Flow Cytometric Fractionation of Fetal Mouse Liver Cells
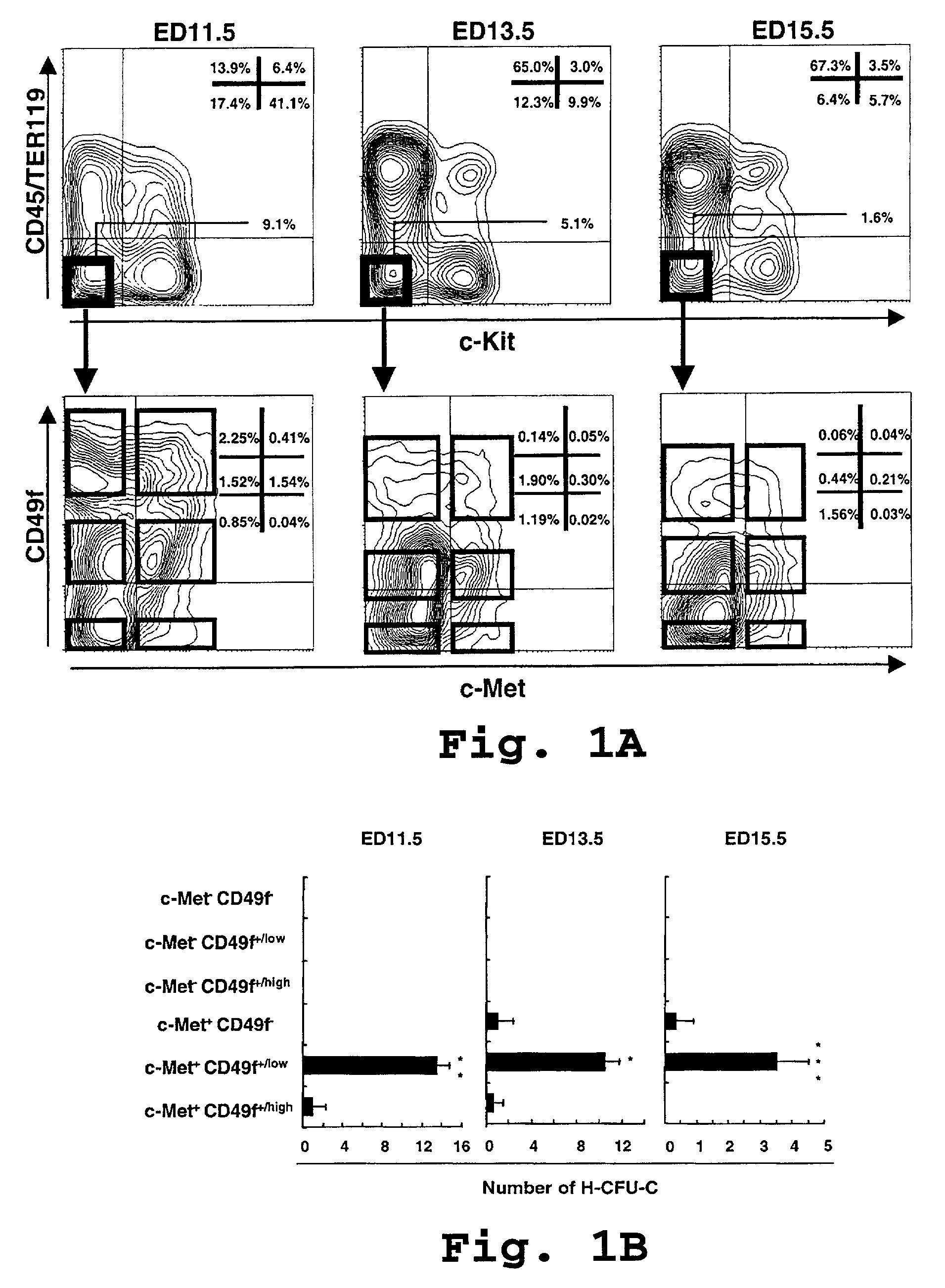
[0102]In this example, we fractionated H-CFU-H according to the method that we have previously reported (Suzuki et al., 2000), and thereafter we attempted to sort for cells expressing c-Met, the hepatocyte growth factor (HGF) receptor for further fractionation.
(Fractionation of “H-CFU-C” by Flow Cytometry) Cell preparation
[0103]Single cell suspensions of liver cells were prepared from Balb / cA ED13.5 fetal mice (CLEA, Tokyo, Japan). For sequential analysis, we used embryonic day (ED) 11.5, 13.5, 15.5, and neonatal (1 day after birth) mice. For sorting, we used mechanical pipetting in staining medium (PBS containing 3% FBS). Cell viability after each treatment was exceeded 90% as assessed by trypan blue dye exclusion.
Cell Staining and FACS Analysis
[0104]Dissociated liver cells were incubated at 4° C. for 30 minutes with biotinylated anti-CD45 and TER119 mAb (PharMingen, San Jose, Calif.), and anti-c-Met mAb (Upstate Biotechnology...
example 2
Characterization of c-Met+ CD49f+ / low c-Kit− CD45− TER119− cells
[0113]In the present example, we characterized of c-Met+ CD49f+ / low c-Kit− CD45− TER119− cells.
[0114]High enrichment in H-CFU-C achieved in Example 1 permitted efficient culture of clone-sorted c-Met+ CD49f+ / low c-Kit− CD45− TER119− cells for analyses of self-renewal and differentiation potential.
[0115]For low density culture analysis, sorted cells were plated on laminin-coated 6-well plates (Becton Dickinson) at a density of 30 cells / cm2 and cultured in our fresh standard medium (1:1 mixture of DMEM and F-12 (Sigma, Chemical Co., St. Louis, Mo.) with 10% fetal bovine serum (JRH BIOSCIENCES, Lenexa, Kans., γ-insulin (1 μg / ml) (Wako, Tokyo, Japan), dexamethasone (1×10−7 M) (Sigma), nicotinamide (10 mM) (Sigma), L-glutamine (2 mM) (Gibco BRL, Gaithersburg, Md.), β-mercaptoethanol (50 μM) (Sigma), HEPES (5 mM) (Wako), and penicillin / streptomycin (Gibco BRL)). For single cell culture analysis, we used standard medium 50% su...
example 3
Clonal Expansion and Self-Renewal Capability of H-CFU-C in Culture
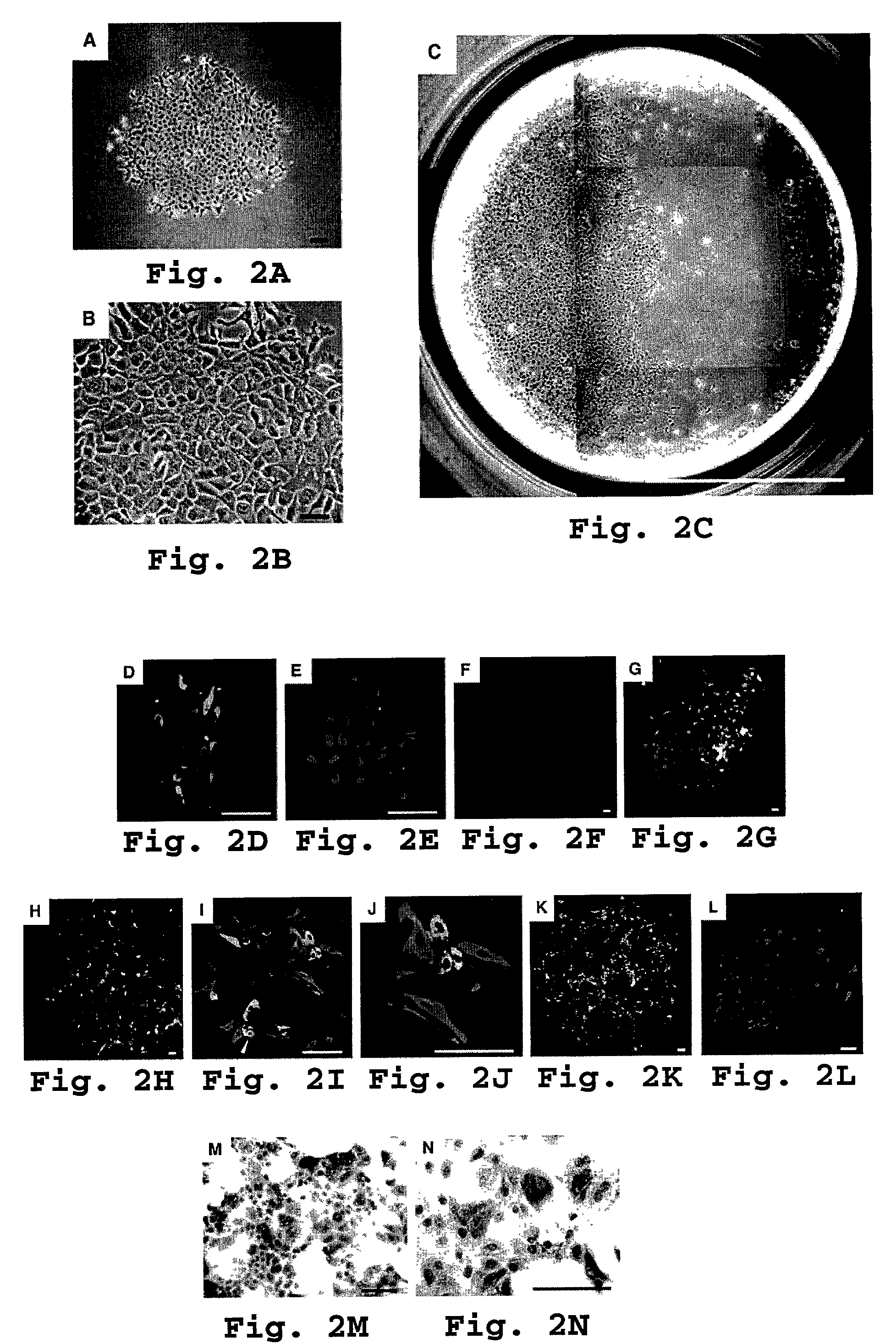
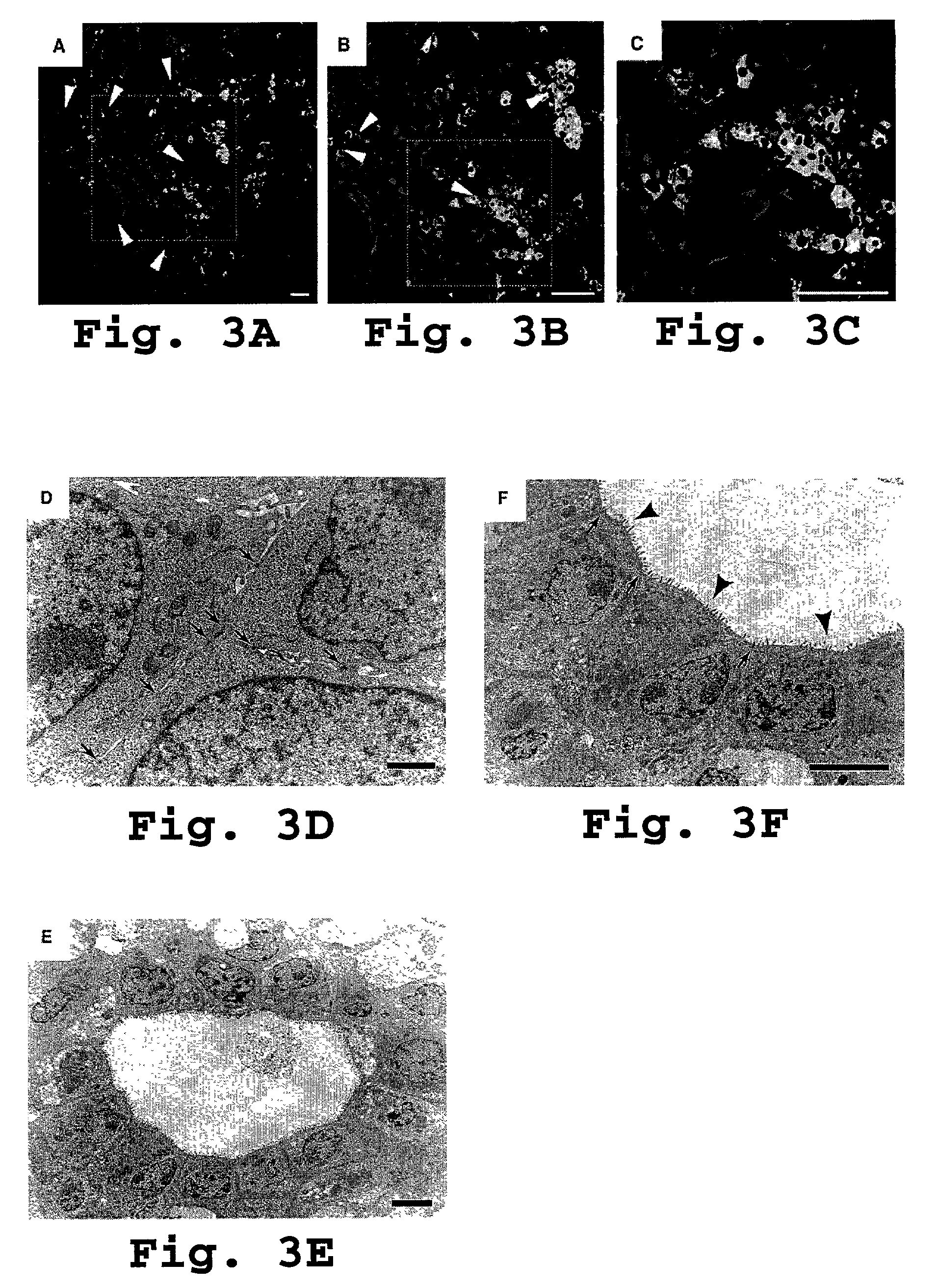
[0121]We next used sub cloning experiments to test the self-renewal potential of H-CFU-C. Single c-Met+ CD49f+ / low c-Kit− CD45− TER119− cells were clone-sorted and individually cultured. This yielded several large H-CFU-C colonies (see FIG. 2 C), which we then replated onto new culture dishes. Over 2 to 3 weeks, about half of subcultured clones gradually expanded, finally to become confluent. These clonally expanding subcultured cells then again underwent clone-sorting and single cell culture.
[0122]We then conducted detection of hepatocyte or cholangiocyte marker gene expression by RT-PCR as described previously in Suzuki at al., 2000. Briefly, a cloning ring (Iwaki Glass, Tokyo, Japan) was placed on the colony and total RNA was prepared by gentle pipetting cells with ISOGEN (Nippon Gene, Tokyo, Japan). Prior to reverse transcription, 0.8 μl of oligo-d(T)1218 primers was added to the total RNA solution. The reaction m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com