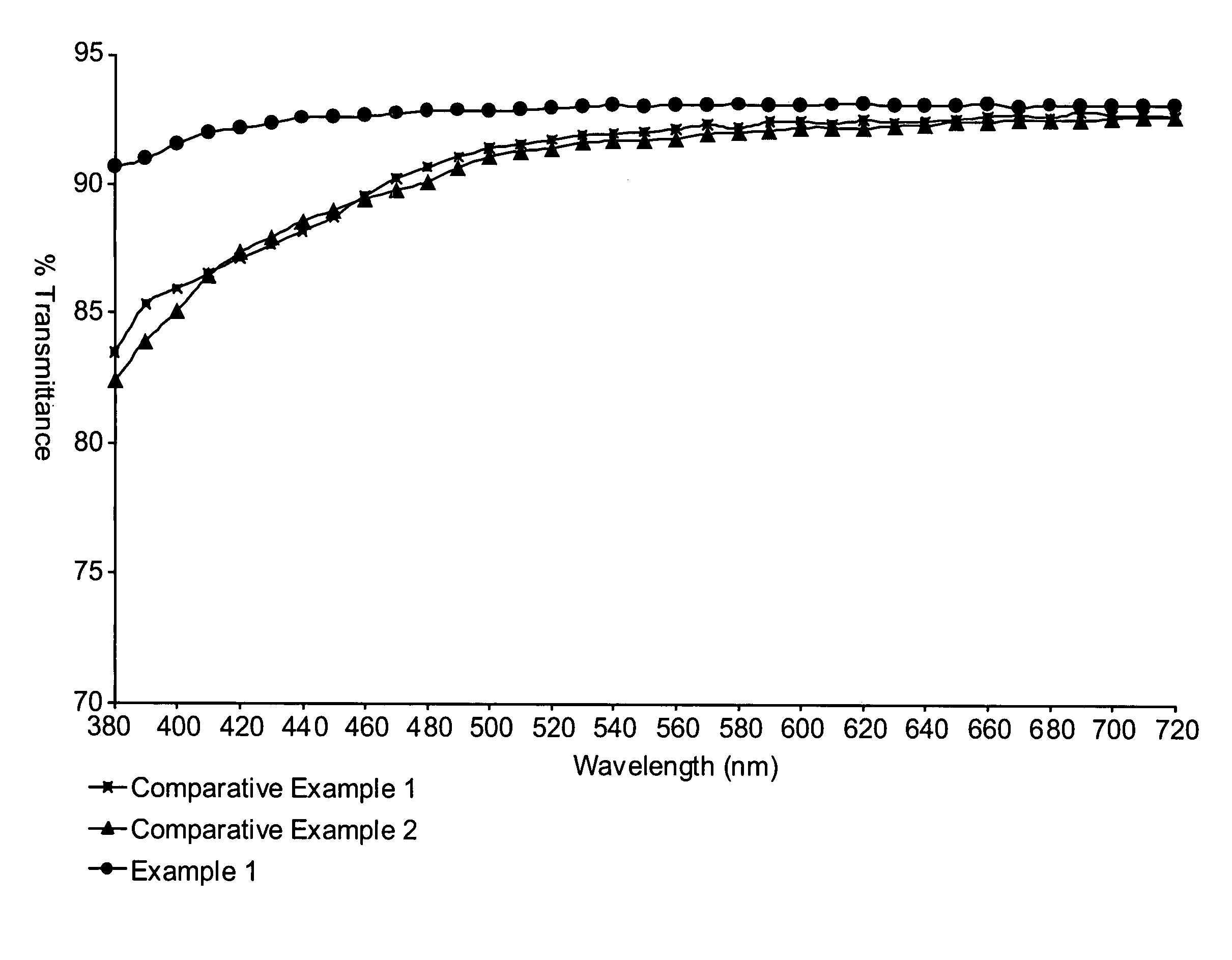Curable thiol-ene compositions for optical articles
a technology of thiol-ene compositions and optical articles, applied in the field of curable thiolene compositions, can solve the problems of optical defects, unpredictability of registration, net shrinkage in volume, etc., and achieve the effects of low birefringence, low shrinkage, and rapid photocuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
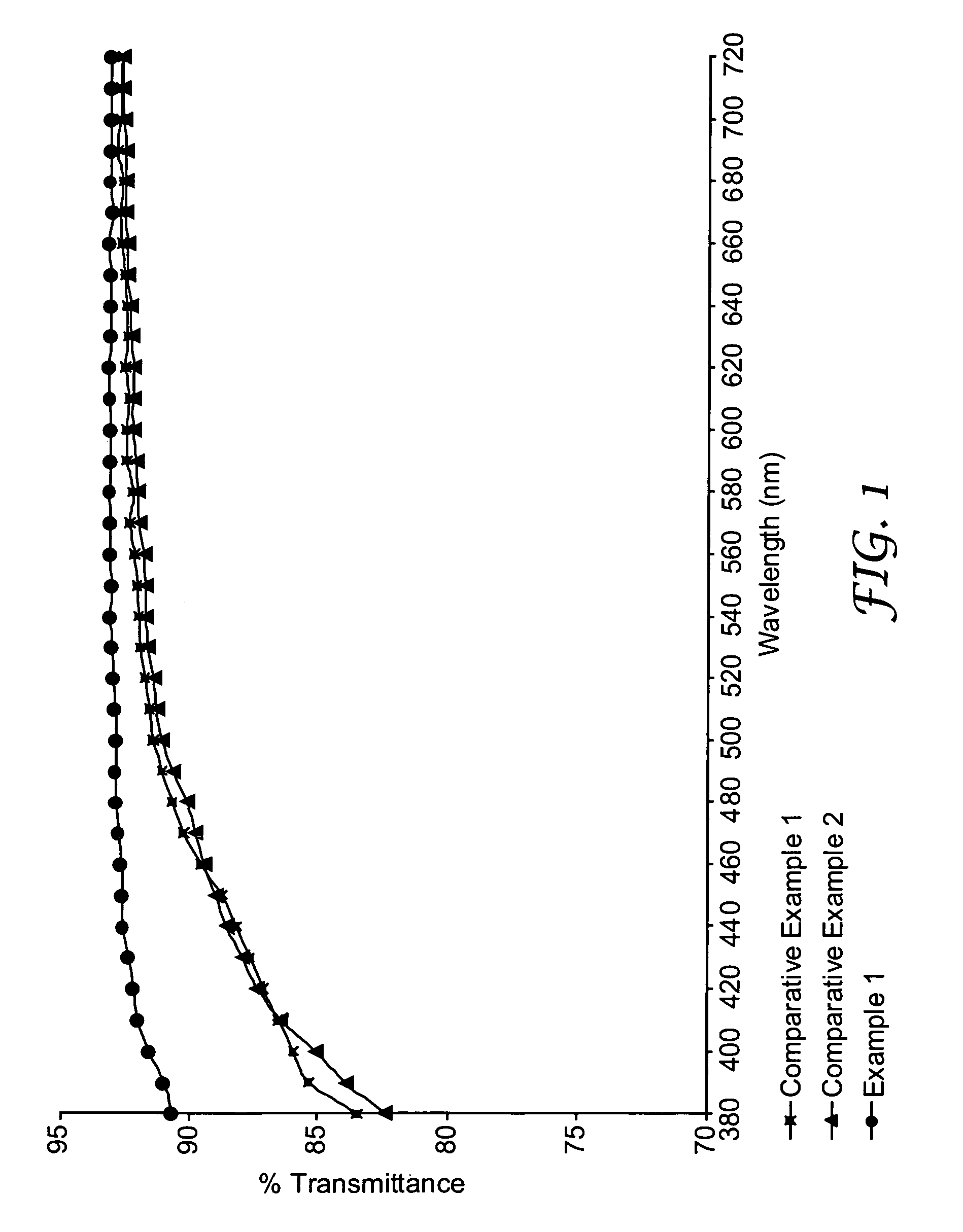
Examples
examples
[0064]These examples are for illustrative purposes only and are not meant to be limiting on the scope of the appended claims. All parts, percentages, ratios, etc. in the examples and the rest of the specification are by weight, unless noted otherwise. Solvents and other reagents used were obtained from Sigma-Aldrich Chemical Company; Milwaukee, Wis. unless otherwise noted.
[0065]
Table of AbbreviationsAbbreviation ofTrade NameDescriptionPETMPPentaerythritol tetrakis(3-mercaptopropionate) availablefrom Dow Chemical Company, Midland, MITMPTMPTrimethylolpropane tris(3-mercaptopropionate)available from Dow Chemical Company, Midland, MICN1963Aliphatic urethane methacrylate oligomer containing25% TMPTMA as reactive diluent available fromSartomer Company Inc, Exton, PAPro7327Aliphatic urethane methacrylate oligomer containing noreactive diluent (i.e. CN1963 without TMPTMA)available from Sartomer Company Inc, Exton, PATMPTMATrimethylolpropane trimethacrylate, SR350, availablefrom Sartomer Com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tg | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| shrinkage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com