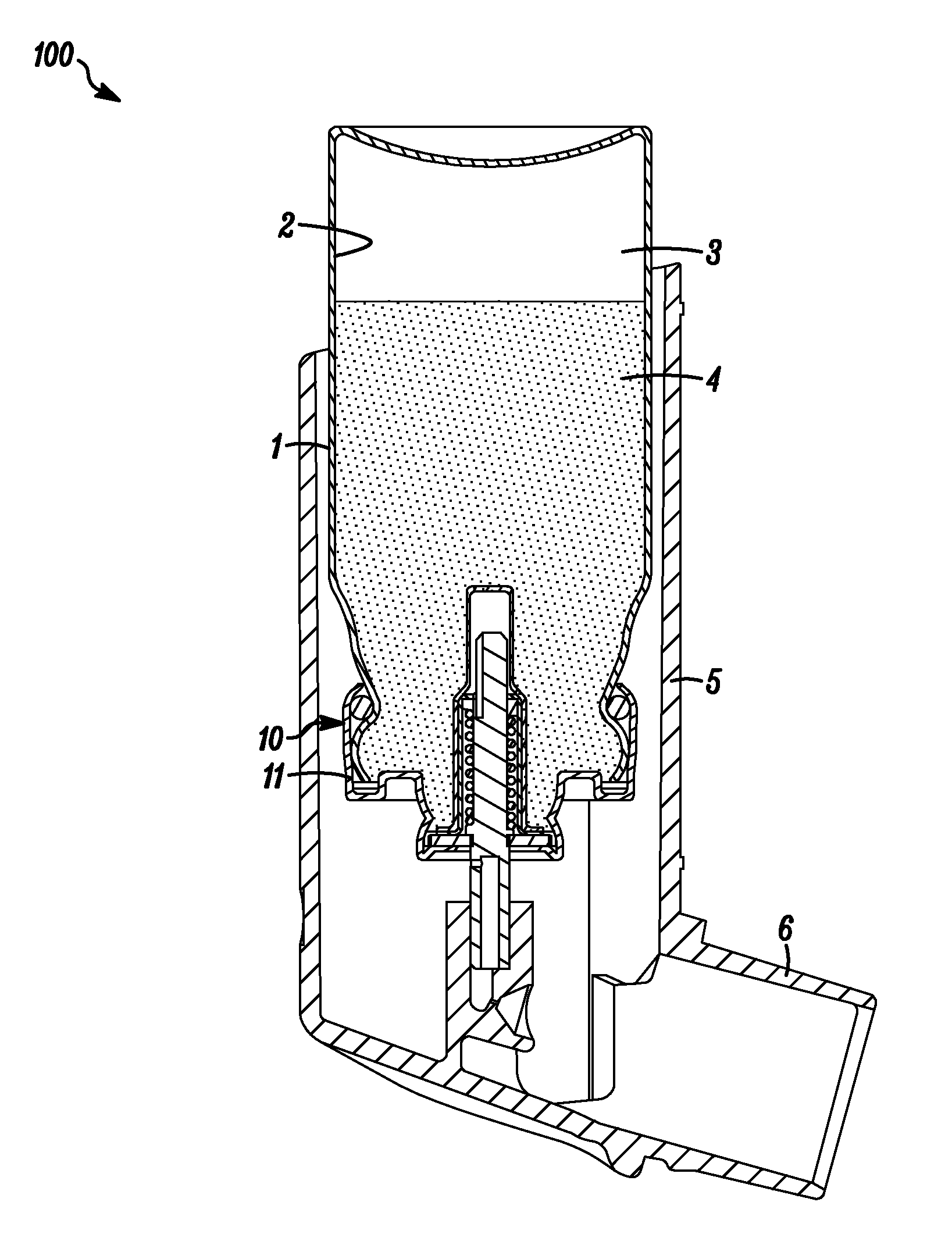
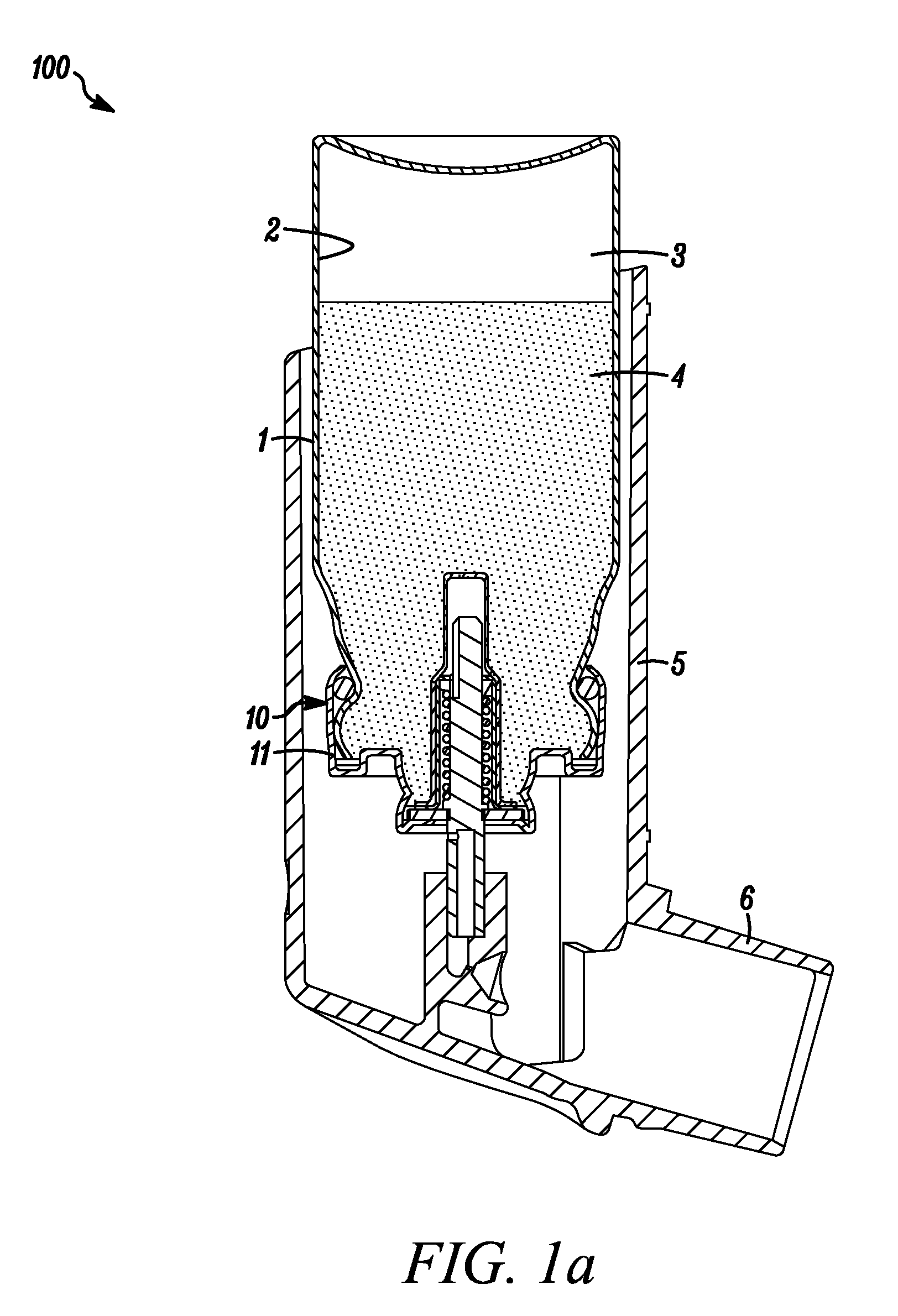
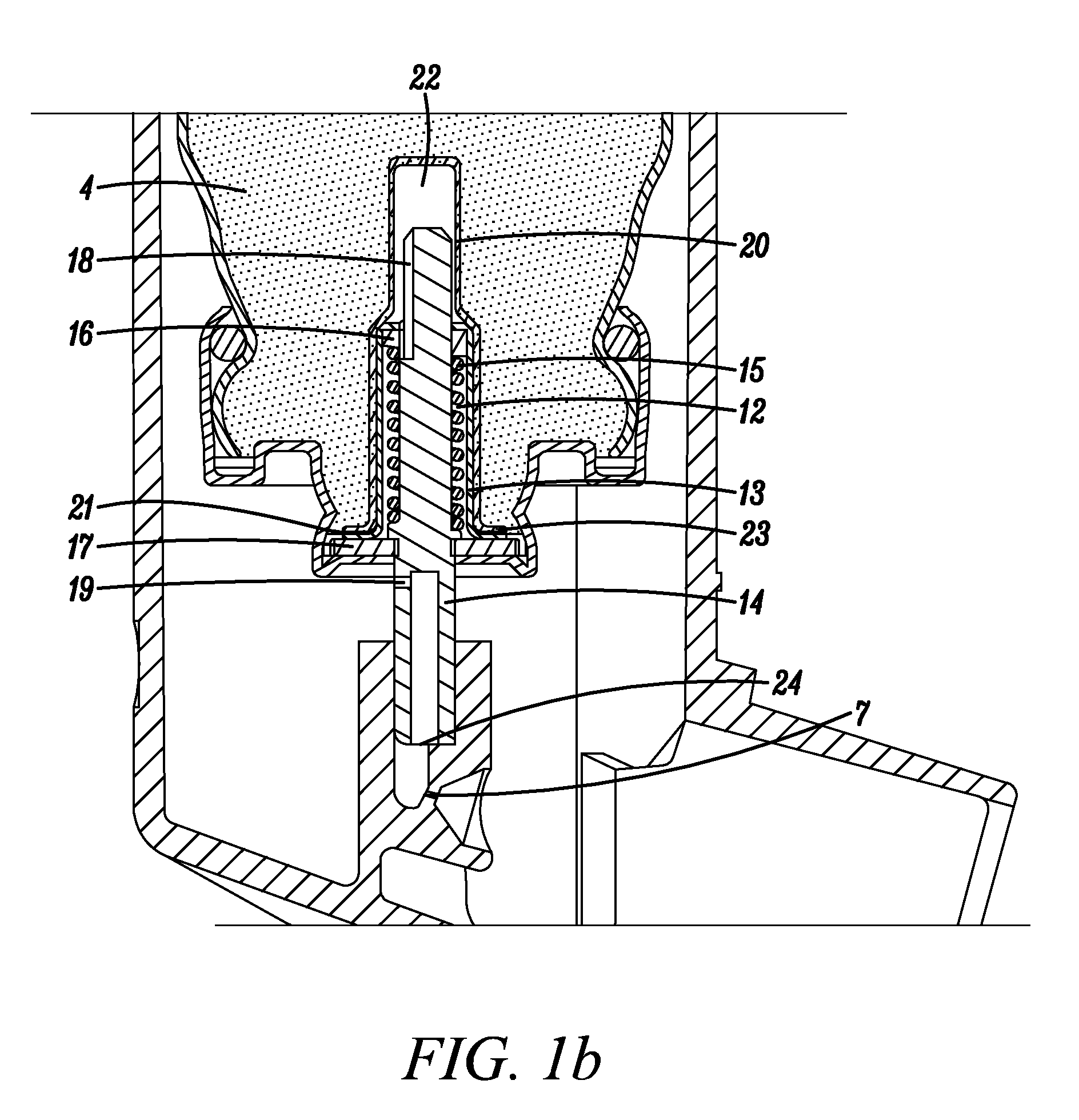
Medicinal inhalation devices and components thereof
a technology of inhalation device and component, which is applied in the direction of packaging foodstuffs, instruments, packaged goods, etc., can solve the problems of undesirable effects, adsorption of ambient water, and unsuitable material for a particular component, and achieve enhanced stability and/or resistance to attack, enhanced stability and resistance of the applied polyfluoropolyether-containing coating to attack, and flexural strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Exemplary Silane Treatment Methods
[0164]The following are exemplary silane treatment methods:
Method A:
[0165]Place a solution (3 liters (L)) of 0.1% (w / w) C3F7O(CF(CF3)CF2O)pCF(CF3)—C(O)N(H)(CH2)3Si(OCH3)3 (average value of p is 10, average MW of silane is about 2151, and fraction of silane with a polyfluoropolyether segment having a weight average MW lower than 750 is zero), 0.6% (w / w) tetraethoxysilane and 1% (w / w) acetic acid in HFE-7200 fluid (available from 3M Company, St. Paul, Minn. under the trade designation “NOVEL HFE-7200”) in a 4-L beaker at room temperature, and place beaker in a dip coater. Fix component to be coated vertically above the solution, introduce component into the solution at a controlled rate of 15 millimeters per second (submerging the component entirely in the solution) and hold in place for at least five seconds. Subsequently withdrawn component from the solution at a controlled rate of 15 millimeters (mm) per second and allow to drain. After draining, p...
examples 1 to 3
[0169]Standard deep drawn aluminum MDI containers having a nominal volume of 10 milliliters are washed with trichloroethylene and coated in accordance with Method A (Example 1), Method B (Example 2) and Method C (Example 3), respectively. Containers are fitted with metered dose valves of the type marketed under the trade designation SPRAYMISER (3M Company, St. Paul, Minn., USA) having a 50 mcl metering chamber and then a formulation consisting of 1.97 mg / ml albuterol sulfate (having a majority of particles in the range of 1 to 3 microns) and HFA 134a is pressure-filled into the canisters.
examples 4 to 6
[0170]Standard deep drawn aluminum MDI containers having a volume of 10 millimeters are washed with trichloroethylene, anodized and coated in accordance with Method A (Example 4), Method B (Example 5) and Method C (Example 6), respectively. Containers are fitted with metered dose valves of the type marketed under the trade designation SPRAYMISER (3M Company, St. Paul, Minn., USA) having a 50 mcl metering chamber and then a formulation consisting of 1.97 mg / ml albuterol sulfate (having a majority of particles in the range of 1 to 3 microns) and HFA 134a is pressure-filled into the canisters.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com