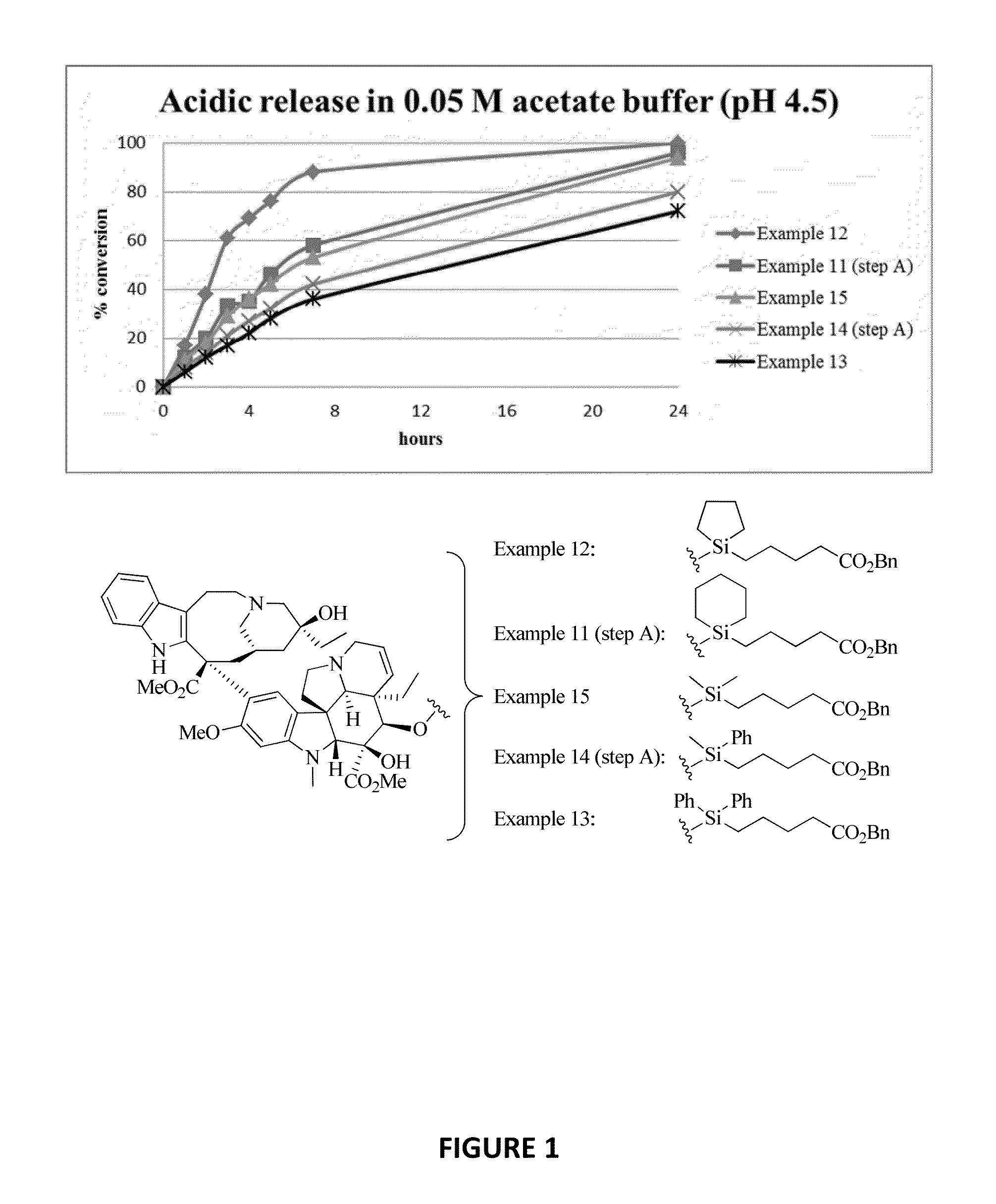
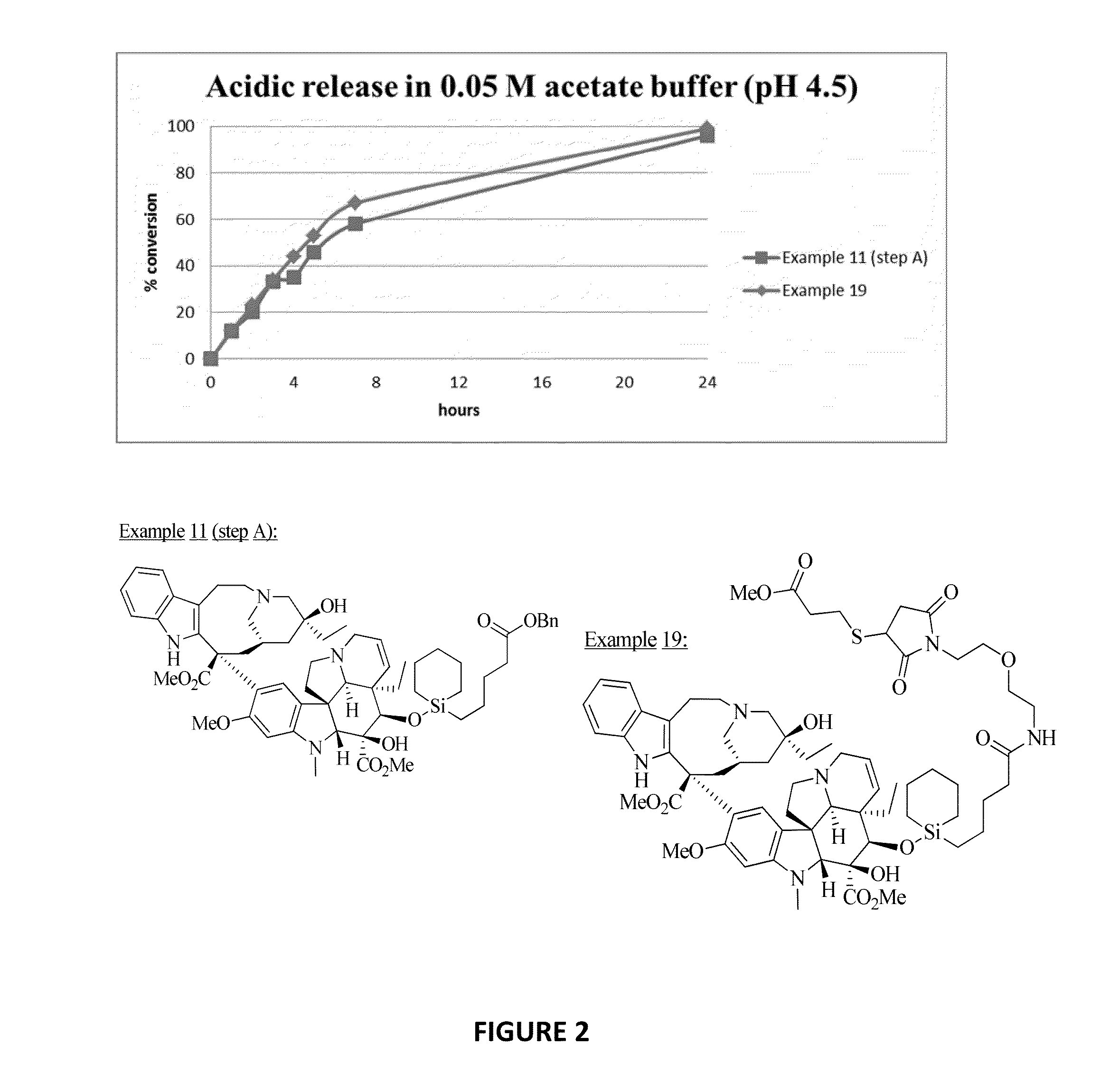
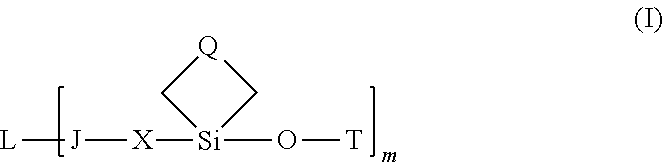
Ligand-therapeutic agent conjugates, silicon-based linkers, and methods for making and using them
a technology of ligand-therapeutic agent and conjugate, which is applied in the field of conjugate compounds and silicon linker compounds, can solve the problems of being too labile to achieve the long plasma half-lives desired for the intact conjugate, and achieve the effect of long plasma half-lives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedures
[0393]Unless otherwise noted, reagents and solvents were used as received from commercial suppliers. Proton nuclear magnetic resonance (NMR) spectra were obtained on Bruker spectrometers at 300 or 500 MHz. Spectra are given in ppm (δ) and coupling constants, J, are reported in Hertz. Tetramethylsilane (TMS) was used as an internal standard. Mass spectra were collected using a Waters SQ Detector single quadripole mass spectrometer (ESI). High performance liquid chromatograph (HPLC) analyses were obtained using a Luna C18(2) column (250×4.6 mm, Phenomenex, Torrance, Calif.) with UV detection at 254 nm using the standard solvent gradient programs Method A or Method B:
[0394]
Method A:TimeFlow(min)(mL / min)% A% B0.01.090.010.020.01.00.0100.025.01.00.0100.027.01.090.010.032.01.090.010.0A = Water with 0.1% Trifluoroacetic AcidB = Acetonitrile with 0.1% Trifluoroacetic Acid
[0395]
Method B:TimeFlow(min)(mL / min)% A% B0.0880.020.010.01.00.0100.012.01.00.0100.014.01.080.020.016.0...
example 2
Preparation of benzyl 5-(chlorodimethylsilyl)pentanoate
[0396]
[0397]To a mixture of chlorodimethylsilane (0.730 g; 6.57 mmol) and benzyl pent-4-enoate (1.25 g; 6.57 mmol) under argon was added one drop of Karstedt's catalyst solution (in xylenes; ˜2% Pt). The reaction vessel was sealed and the mixture was heated to 60° C. for 13.5 hours. The crude liquid was used without purification.
example 3
Preparation of benzyl 5-(chlorodiphenylsilyl)pentanoate
[0398]
[0399]To a mixture of chlorodiphenylsilane (0.49 g; 2.58 mmol) and benzyl pent-4-enoate (0.50 g; 2.58 mmol) under argon was added two drops of Karstedt's catalyst solution (in xylenes; ˜2% Pt). The reaction vessel was sealed and the mixture was heated to 60° C. for 15 hours. The crude liquid was used without purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| physiological temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com