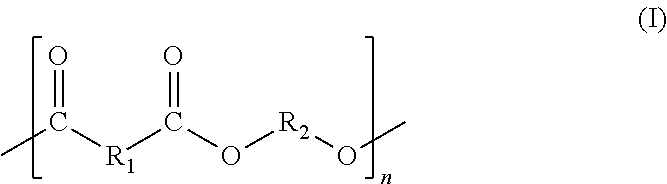
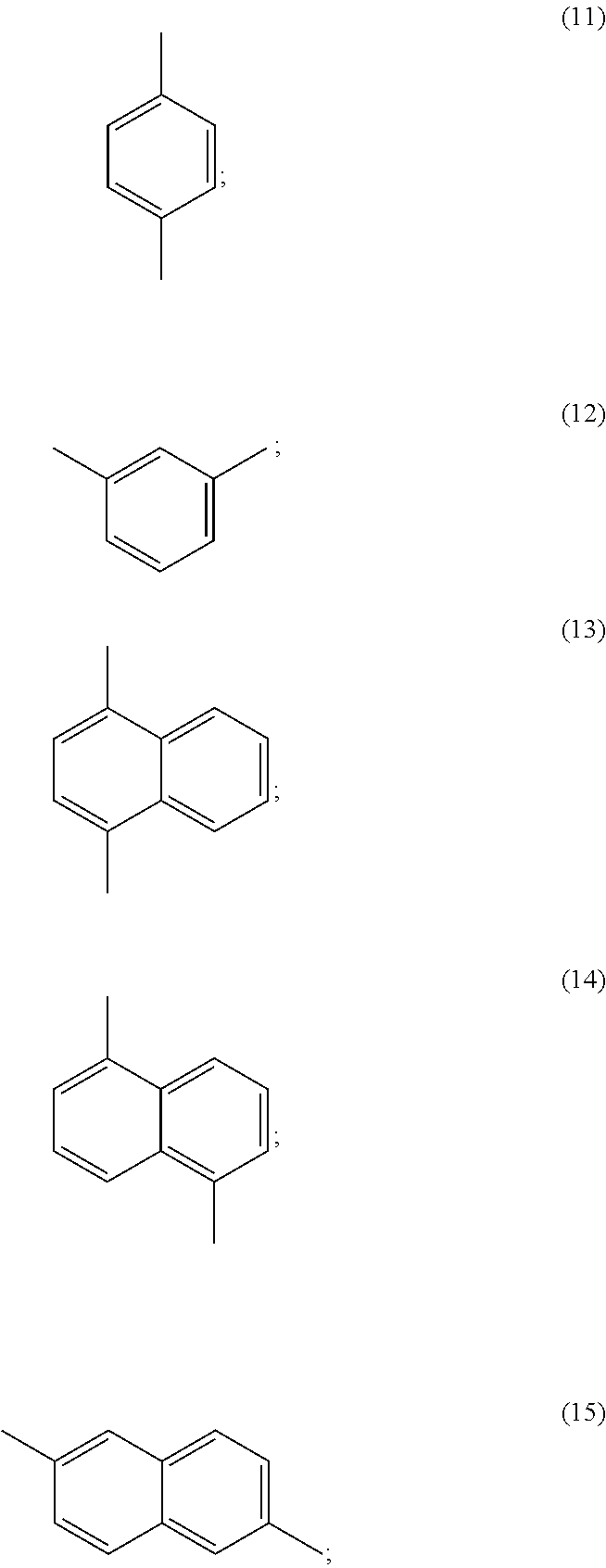
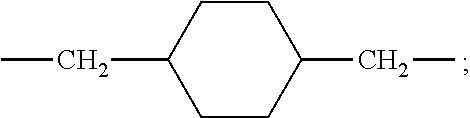
Polylactic acid block copolymers and preparation methods thereof
a technology of polylactic acid block and copolymer, applied in the field of polylactic acid, can solve the problems of limited wide application range, poor crystallization ability of pla, and high brittleness of pla, and achieve the effect of high melting poin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0112]Step 1: The ring-opening polymerization of cyclic poly butylene terephthalate (CBT), that is, the preparation of the macrocyclic poly butylene terephthalate (PBT).
[0113]Under N2, the CBT (10 g) and stannoxane catalyst (0.5 g) were loaded into a flask (100 mL) which was baked, protected under N2 and cooled. The dehydrated and de-oxygened o-dichlorobenzene was added into the flask by a syringe and the concentration of monomer CBT was 2 kg / L. The reaction was carried out at 180° C. and monitored by GPC. After 10 minutes, the signal peak of CBT disappeared and the reaction was quenched. The obtained product was cooled and filtered, and washed with dried CHCl3 to wash away the residual monomer CBT. The product was dried at 60° C., and the PBT containing catalyst was obtained.
[0114]Step 2: The polymerization of lactide initiated by the PBT containing catalyst.
[0115]Under N2, the PBT (0.5 g) prepared in step 1 and L-lactide (5.0 g) were added into a dried and de-oxygened flask (100 m...
example 2
[0123]Step 1: The ring-opening polymerization of cyclic poly ethylene terephthalate (CET), which was the preparation of the macrocyclic poly ethylene terephthalate (PET).
[0124]Under N2, the CET (10 g) and stannoxane catalyst (0.05 g) were loaded into a flask (100 mL) which was baked, protected under N2 and cooled. The dehydrated and de-oxygened solvent chlorobenzene was charged into the flask by a syringe and the concentration of monomer CET was 1 kg / L. The reaction was carried out at 120° C. and was monitored by GPC. After 48 h, the signal of CET disappeared, and then the reaction was quenched. The system was cooled to room temperature. After that, the product was filtered and washed with dried CHCl3, and the residual monomer CET was washed away. The product was vacuum dried at 60° C., and the PET containing catalyst was obtained.
[0125]Step 2: The polymerization of lactide which was initiated by the PET containing catalyst.
[0126]Under N2, the PET (0.5 g) obtained in step 1 and D-la...
example 3
[0129]Step 1: the ring-opening polymerization of cyclic poly butylene isophathlate, which was the preparation of the macrocyclic polybutylene isophathlate.
[0130]Under N2, the cyclic poly butylene isophathlate (10 g) and stannoxane catalyst (0.1 g) were loaded into a flask (50 mL) which was baked, protected under N2 and cooled. The mixed solvent of o-dichlorobenzene and chlorobenzene of which V:V=2:1 was charged into the flask by a syringe and the concentration of monomer cyclic poly butylene isophathlate was 2 kg / L. The reaction was carried out at 180° C. and was monitored by GPC. After 10 minutes, the signal of cyclic butylene isophathlate disappeared, and then the reaction was quenched. The system was cooled to room temperature. After that, the product was filtered and washed with dried CHCl3, and the un-reacted monomer was washed away. The product was vacuum dried at 60° C., and the polybutylene isophathlate containing catalyst was obtained.
[0131]Step 2: the polymerization of lac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com