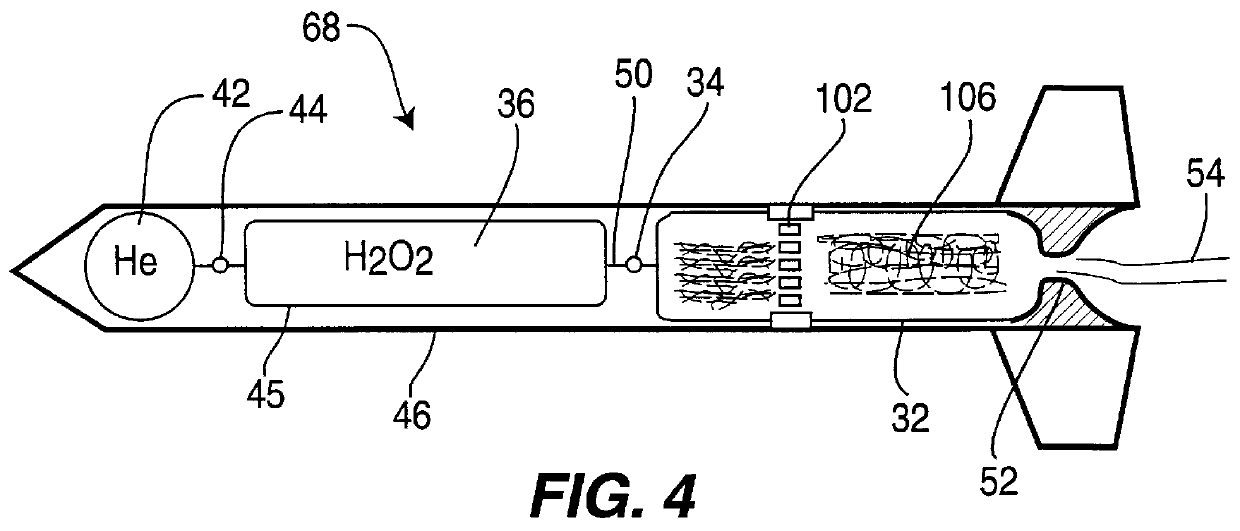
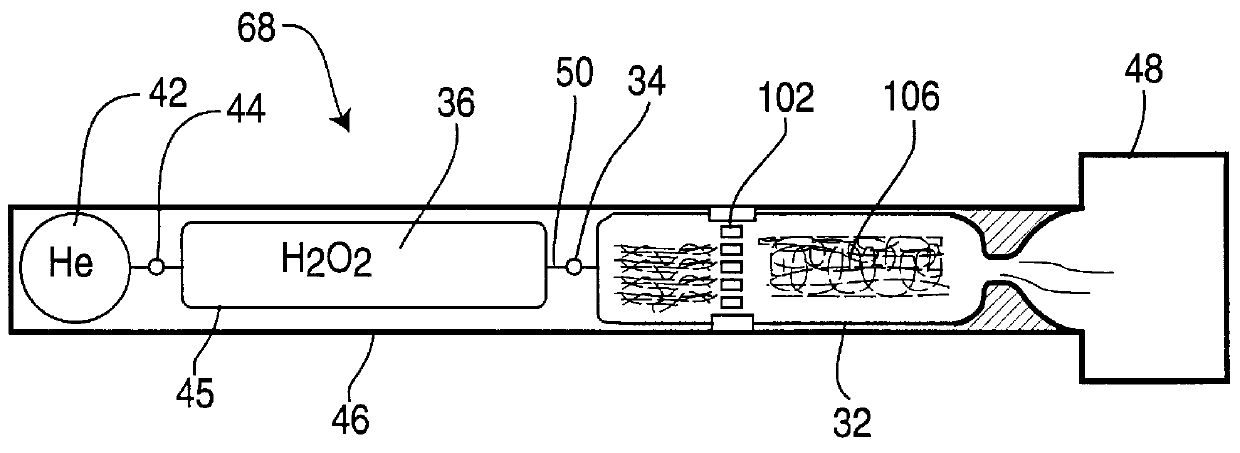
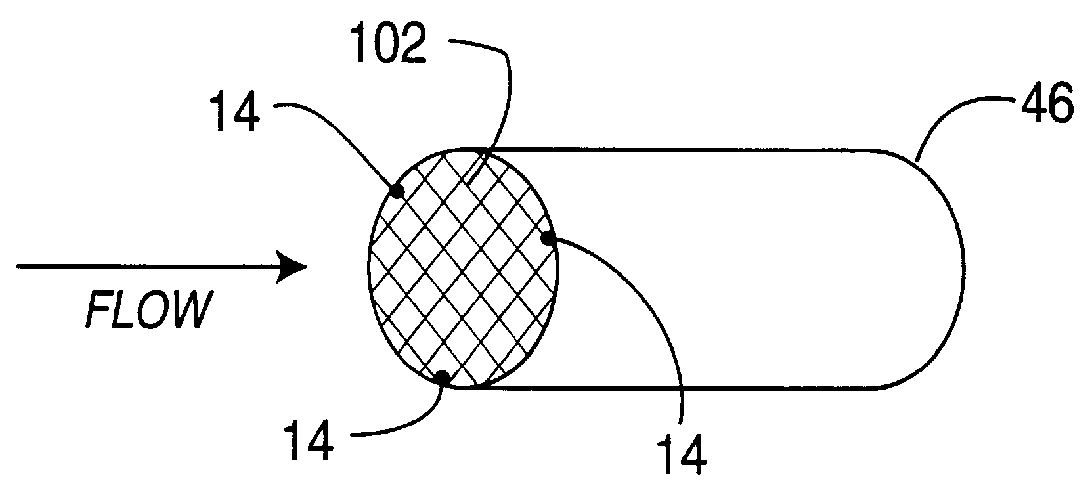
High-activity catalyst for hydrogen peroxide decomposition
a hydrogen peroxide and catalyst technology, applied in the field of high-activity hydrogen peroxide decomposition, can solve the problems of increasing the activity, increasing the pressure drop, and contaminants within the hydrogen peroxide may also plate out onto the silver screen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
1 molar solution of sodium pennanganate was prepared by dissolving 141.93 grams of NaMnO.sub.4 in 1 liter of deionized water. After dissolution, one mole of sodium hydroxide (40.00 grams) was added to the solution and allowed to dissolve to form the impregnation solution. 400, 600, 900, and 1100 pore per in.sup.2 monolithic catalyst substrates, comprised of synthetic cordierite, manufactured by Corning Incorporated of Corning N.Y., were sliced to proper thickness of one-half inch and cored to proper diameter of one inch. These substrates were dried overnight at 200.degree. C. The substrates were allowed to soak in the impregnation solution for one hour, were drained, blotted and then dried and calcined at 325.degree. C. overnight. The impregnation and calcination processes were repeated twice to form the finished catalyst which contained the sodium alkaline promoter in an amount to the active manganese in a molar ratio of two to one.
example 2
1 molar solution of cobalt acetate tetrahydrate was made by dissolving 249.08 grams of Co(C.sub.2 H.sub.3 O.sub.2).sub.2.cndot.4H.sub.2 O in 1 liter of distilled water. After dissolution, one mole of potassium hydroxide (56.11 grams) was added and dissolved to form the impregnation solution. Cored sections of alumina foam, used as the catalytic substrate, were dried overnight at 200.degree. C. The impregnation solution was poured over the substrates, and the entire mass was allowed to soak for one hour at ambient conditions. The substrates were drained, blotted, and then calcined at 250.degree. C. for two hours. The process was repeated once to form the finished catalyst which contained an equimolar ratio of cobalt and potassium, the alkaline promoter.
example 3
0.25 molar solution of potassium permanganate was made by dissolving 39.51 grams of KMnO.sub.4 in 1 liter of demineralized water, forming the impregnation solution. Cylindrical structures of cordierite monoliths of varying pore densities were formed by diamond core drilling, having a nominal diameter of one inch and a nominal length of three inches. After machining, the cylindrical structures were washed and dried overnight at 200.degree. C. The dried billets were soaked in the impregnation solution for two hours, drained, blotted, and then calcined for one hour at 350.degree. C. The impregnation and calcination steps were repeated three times to yield an active catalyst containing an equimolar ratio of manganese and potassium, the alkaline promoter.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com