Pollution-free organic pollutant degradation reagent prepared from reduced metal and application method thereof
An organic pollutant and application method technology, applied in the field of organic pollutant degradation reagents, can solve the problems of weakened hydroxyl radical oxidation ability, time-consuming and laborious post-treatment, and reduced catalytic efficiency, so as to reduce the difficulty of subsequent treatment and reduce iron sludge. The effect of generation and dosage reduction of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
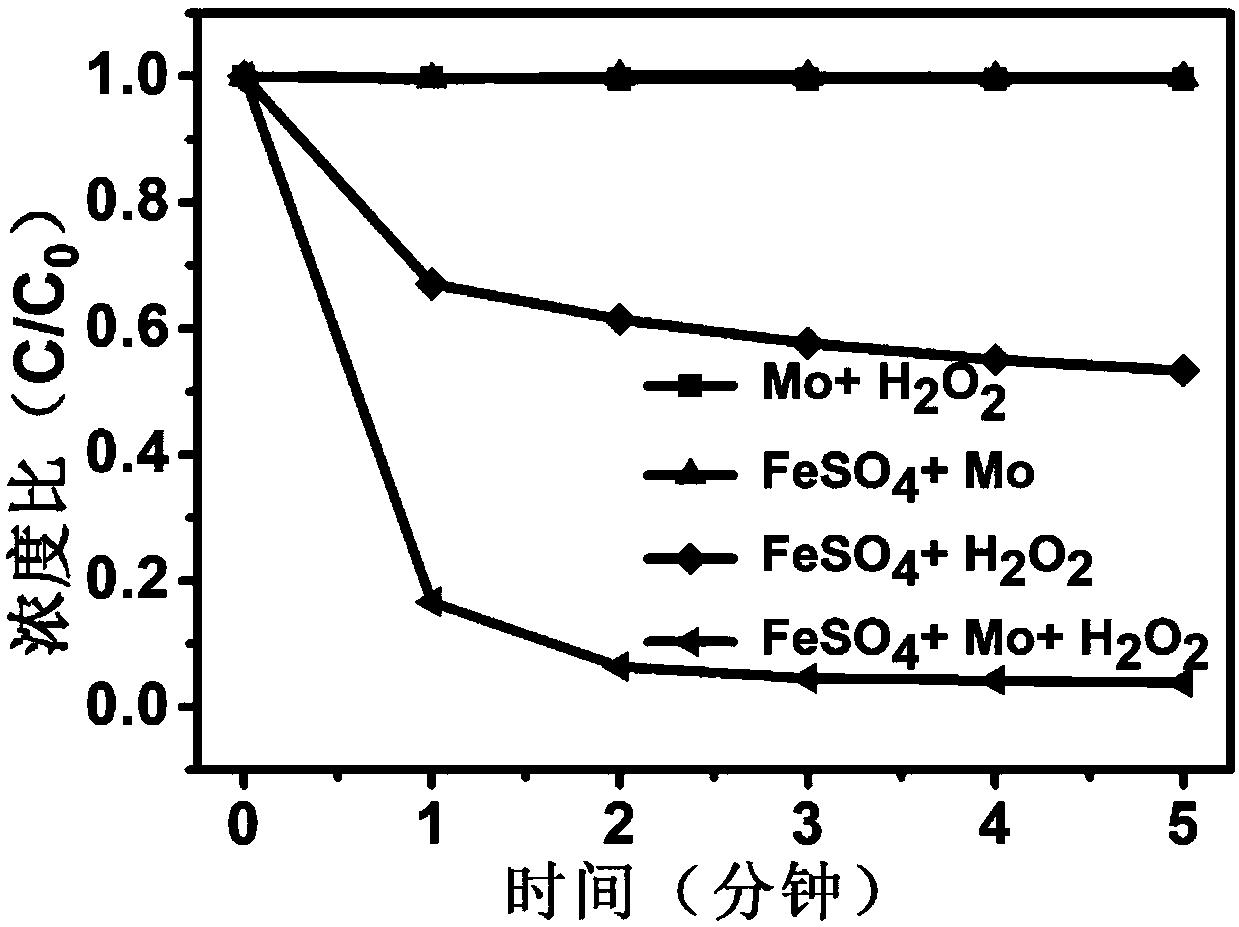
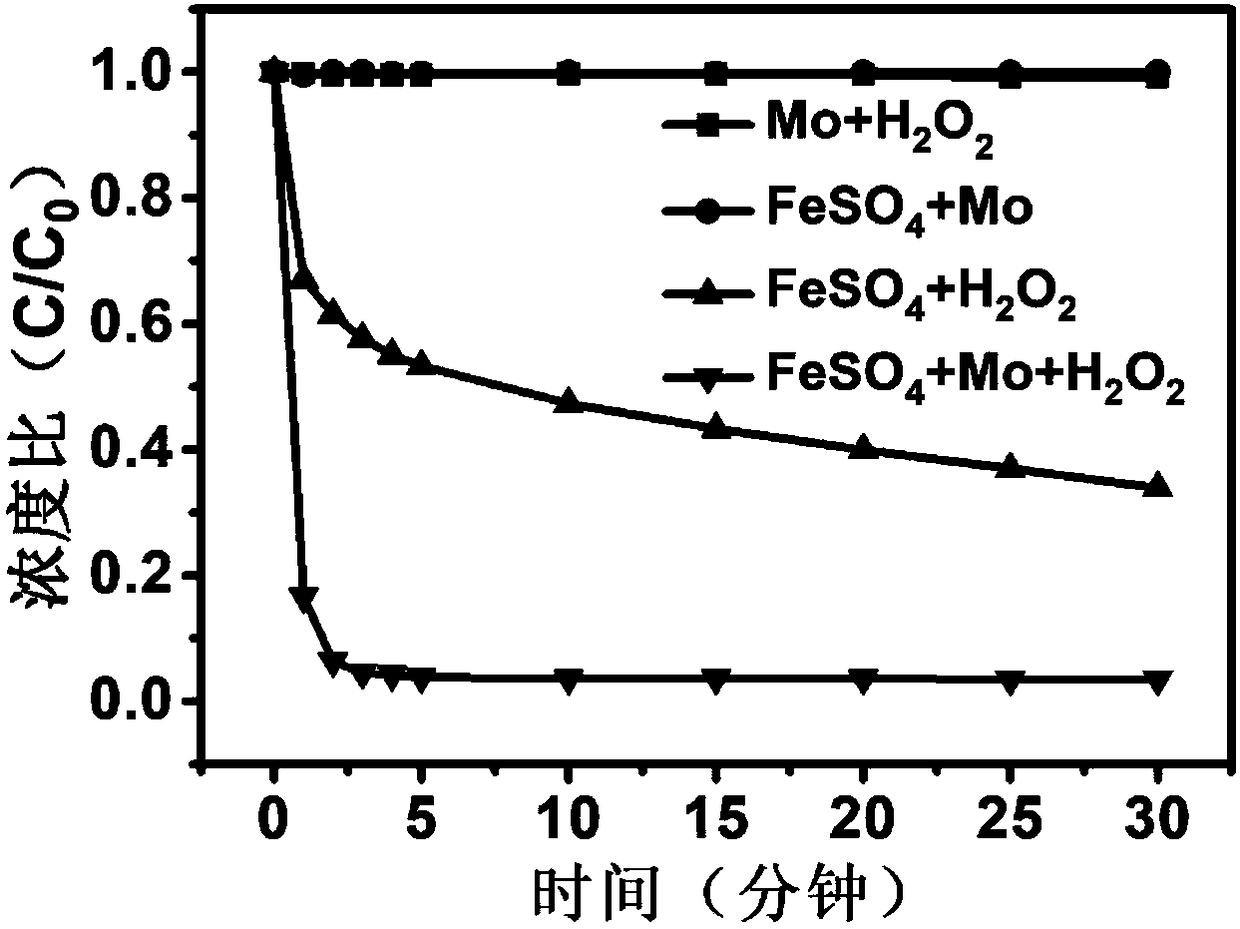
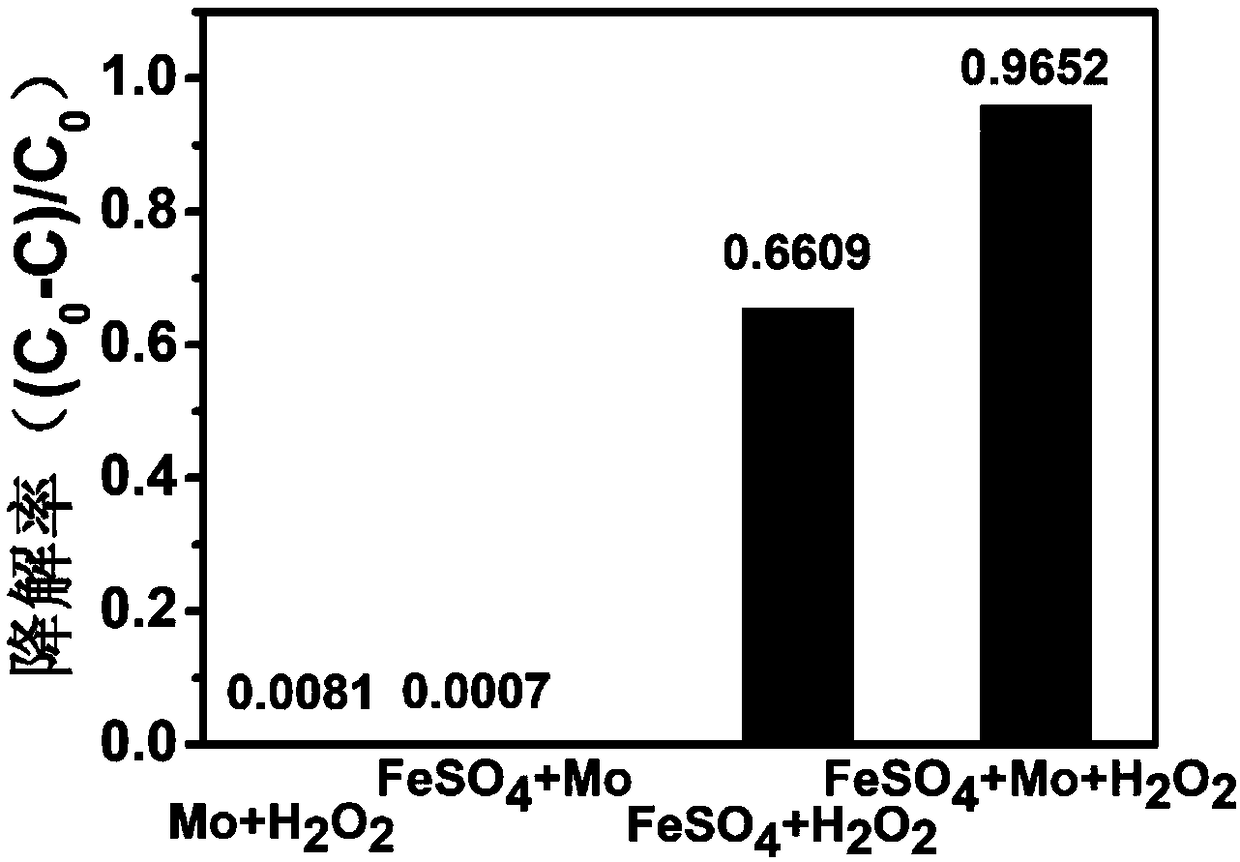
[0047] According to the following four groups of conditions to do the control experiment,
[0048] (1) 10L of waste water to be treated, adjust the pH value to 0-7, FeSO 4 Concentration 0.02g / L, H 2 o 2 Concentration 0.3mmol / L;
[0049] (2) 10L of wastewater to be treated, adjust the pH value to 0-7, Mo concentration 0.05g / L, H 2 o 2 Concentration 0.3mmol / L;
[0050] (3) 10L of waste water to be treated, adjust the pH value to 0-7, FeSO 4 Concentration 0.02g / L, Mo concentration 0.05g / L;
[0051] (4) 10L of waste water to be treated, adjust the pH value to 0-7, FeSO 4 Concentration 0.02g / L, H 2 o 2 Concentration 0.3mmol / L, Mo concentration 0.05g / L;
[0052] Experimental results such as figure 1 , figure 2 , image 3 It can be seen from the experimental results that after adding cocatalyst Mo to the homogeneous Fenton system, the reaction rate of the system and the degradation efficiency of pollutants have been significantly improved.
Embodiment 2
[0054] (1) 10L of waste water to be treated, adjust the pH value to 0-7, FeSO 4 Concentration 0.02g / L, H 2 o 2 Concentration 0.3mmol / L, Mo concentration 0.05g / L;
[0055] (2) Mo powder recovery, washing to remove ionic impurities and dyes on the surface of Mo powder;
[0056] (3) 10L of waste water to be treated, adjust the pH value to 0-7, FeSO 4 Concentration 0.02g / L, H 2 o 2 Concentration 0.3mmol / L, add recovered Mo powder.
[0057] Cocatalyst Mo is recycled 5 times, and the experimental results are as follows Figure 4 As shown, within 10 minutes, the degradation rate of organic pollutants remained above 90%, and as the number of cycles increased, the effect of the cocatalyst Mo did not change significantly, so Mo has good stability as a cocatalyst and can be used in Simple recycling and recycling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com