Purification of cinnamoyl-C-glycoside chromone
a technology of cinnamoyl cglycoside and chromone, which is applied in the field of purification of cinnamoyl cglycoside chromone, can solve the problems of process failure to yield compound 540 of sufficient purity, and conventional methods fail to take into accoun
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
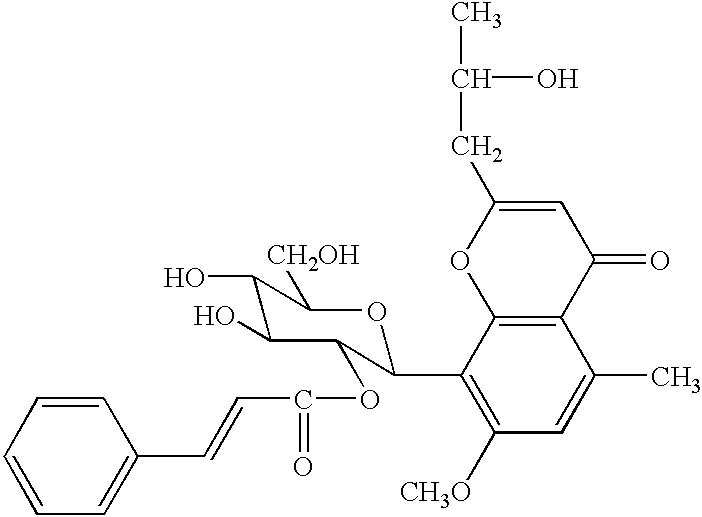
Several commercial processes employed in the processing of slurries from aloe leaf extracts utilize activated carbon (also referred to as activated charcoal) to improve the color of the extracts. Treatment with activated carbon is particularly common with so-called "whole-leaf processing", wherein the entire leaf is ground and processed. A series of experiments on aloe extracts, which had been isolated from the Aloe barbadensis plant, followed by treatment with activated carbon led to the discovery that certain activity associated with the 540 compound was not present in the extracts after treatment with the activated carbon. This led to the discovery that the 540 compound is absorbed by activated carbon. (See, U.S. application Ser. No. 08 / 391,139, entitled Cinnamoyl-C-Glycoside Chromone Isolated from Aloe barbadensis, filed Feb. 21, 1995). A key feature in the method of the present invention is the extraction of compound 540 from decolorizing agents, such as activated charcoal, usi...
second embodiment
In the present invention crude aloe extract, isolated from the leaf rind of the aloe plant is purified by column chromatography without first being treated with a decolorizing agent. This method cuts out a step in the processing of the extracts and provides a fast and simple method of purification, which is ideally suited for large scale production.
In the first embodiment of the present invention, described in Example 1, a decolorizing agent, isolated from the processing of a slurry prepared from aloe leaf extracts, is treated with an organic solvent to extract the absorbed 540 compound. In a preferred embodiment the slurry from which the decolorizing agent is isolated is prepared from the whole leaf or the leaf rind of the Aloe barbadensis plant. Because the majority of the 540 compound is present in the leaf rind or latex, in the most preferred embodiment the slurry is prepared from the leaf rind or latex. In the preferred embodiment the decolorizing agent is activated charcoal, h...
example 1
Extraction of the 540 Compound from Activated Carbon using Methanol and Acetone (room temperature).
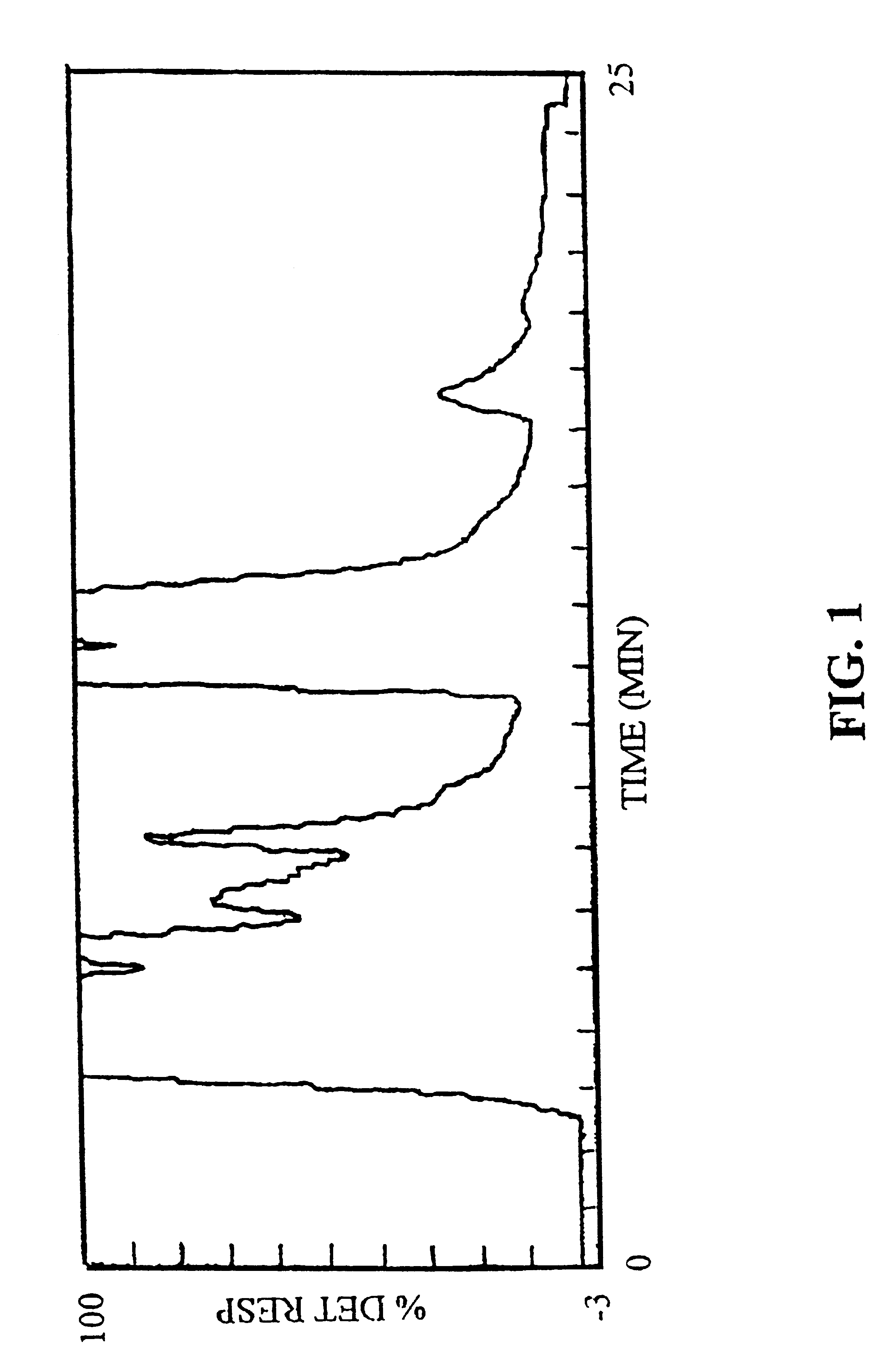
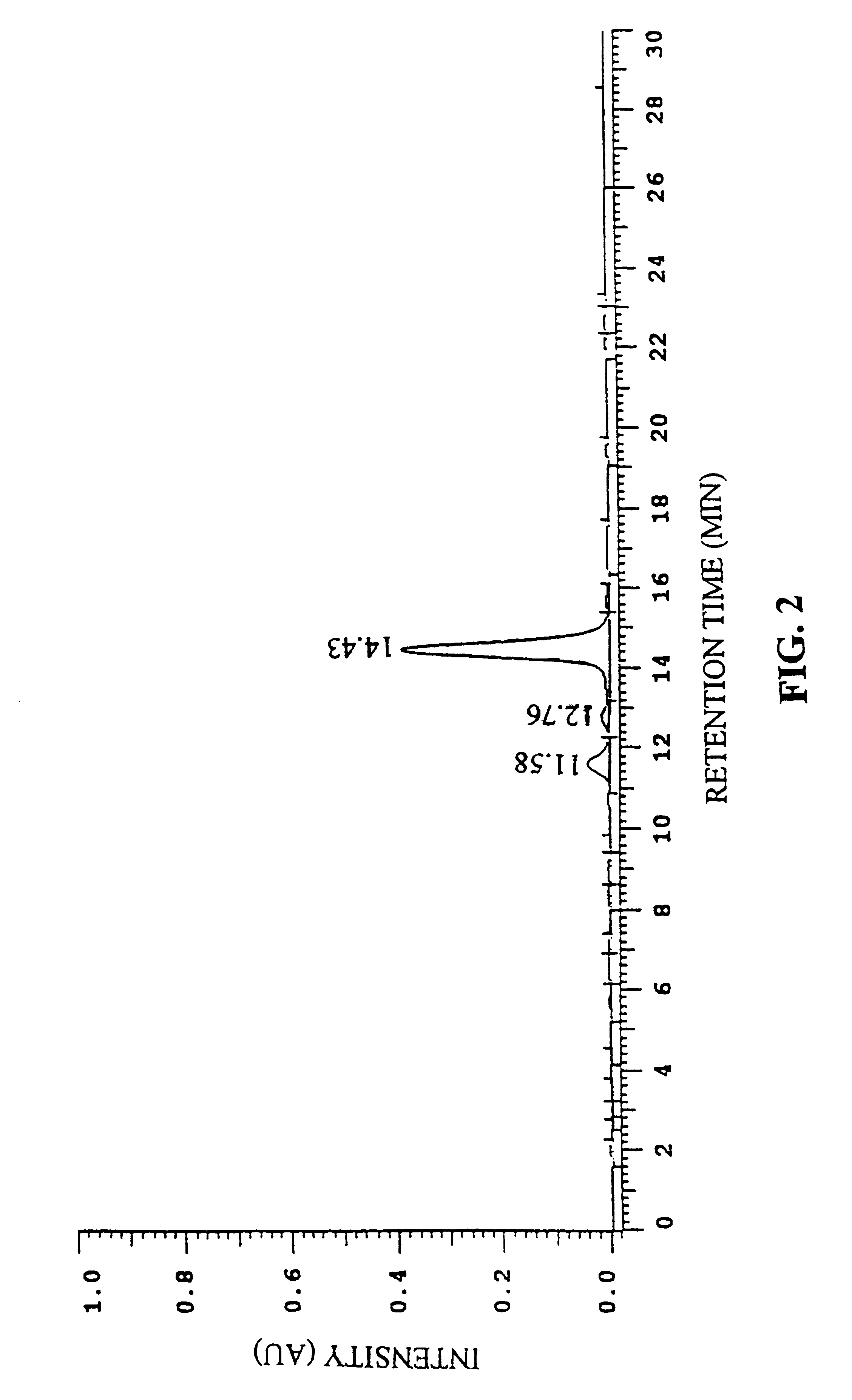
Five hundred grams of freeze-dried, spent activated carbon from whole leaf production was placed in a 2 liter beaker. One and one half liters of methanol was added and the slurry was stored at room temperature for 2 hours. After 2 hours, the slurry was filtered through 0.5 micron cellulose filter paper. The filtrate was stripped of methanol under vacuum and the residue subjected to HPLC analysis. The procedure was then repeated using acetone as the solvent.
Analytical HPLC Analysis
Column: ODS-1, 4 mm.times.250 mm, 5 .mu. particle size
Temperature: Ambient
Mobile Phase: 55% methanol / 45% water
Flow Rate: 0.70 ml / min
Detector Wavelength: 205 nm
Injection Volume: 10-20 .mu.l
Sensitivity: 0.20 AUFS
Retention Time: Approximately 16-17 min.
HPLC analysis shows that methanol is more effective at extracting the 540 compound from the decolorizing agent than acetone. An illustrative HFLC spectrum of the 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com