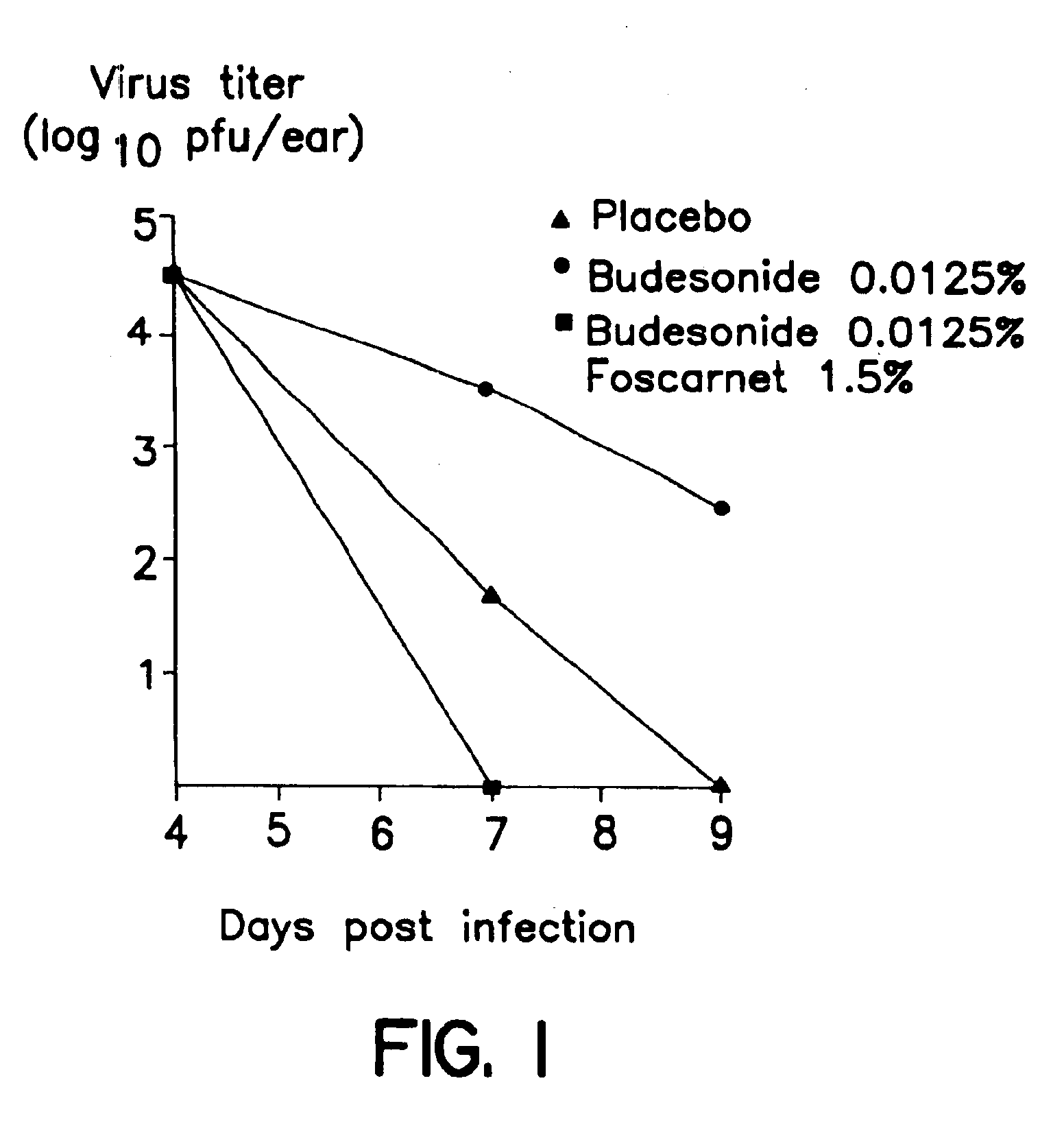
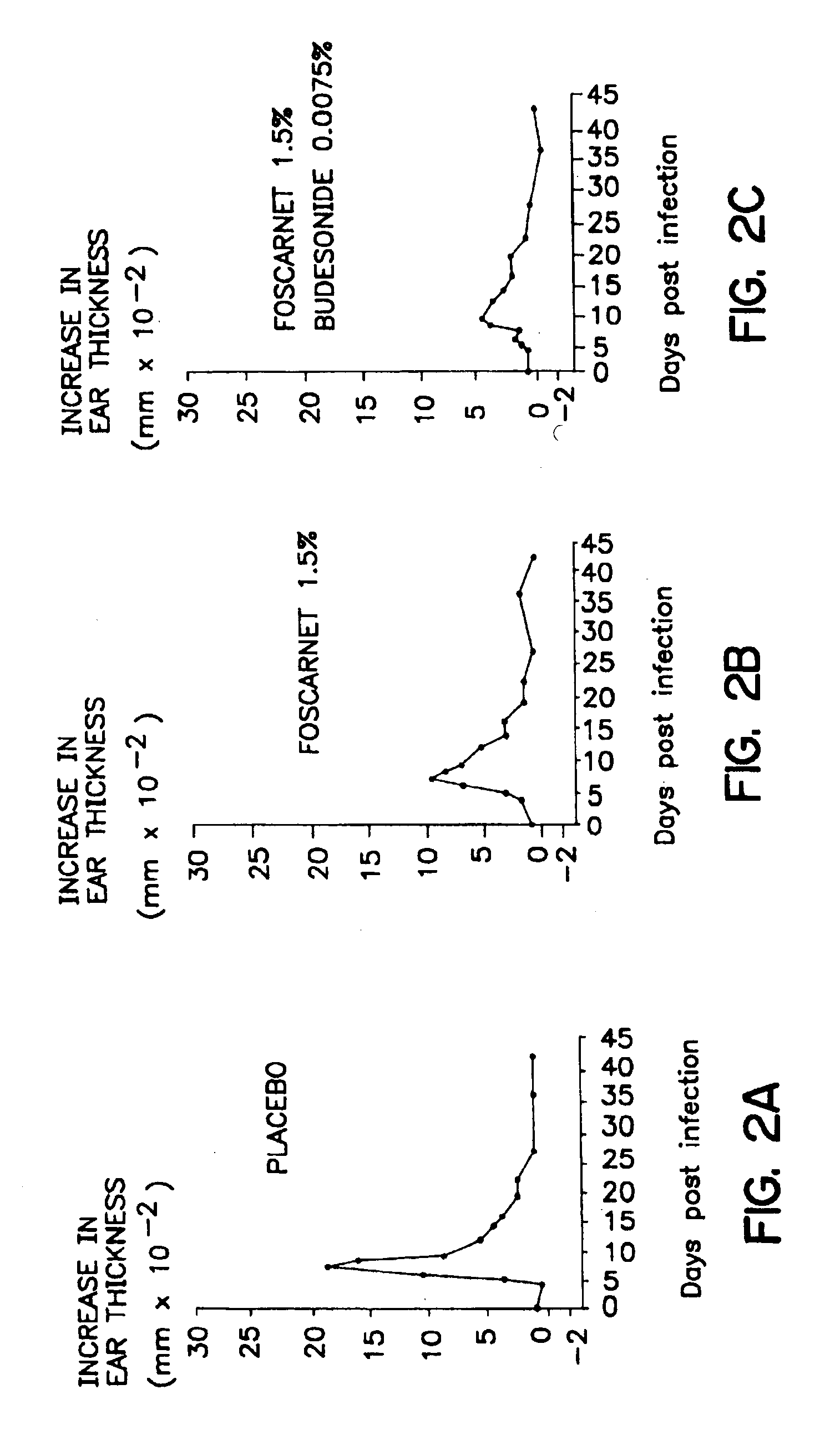
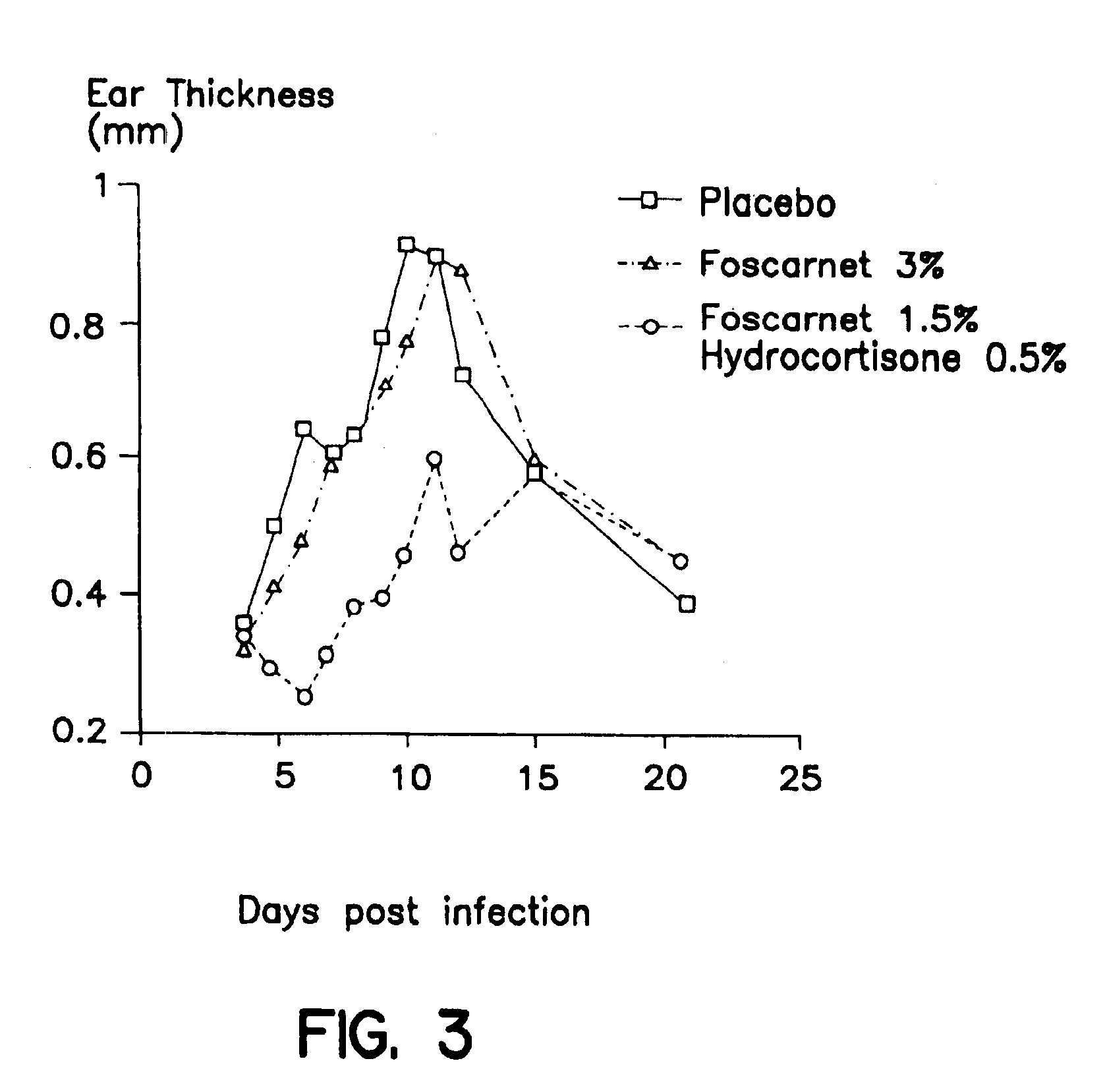
Pharmaceutical combination
a technology of combination and pharmaceuticals, applied in the field of pharmaceutical compositions, can solve the problems of too high systemic toxicity of antiviral compounds to allow skin or mucous membrane application, and achieve the effect of improving the appearance of a human and improving the pharmacological
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Cream of Budesonide 0.0125% and Foscarnet 1.5%
[0052]By simple mixing of Foscarnet Cream and budesonide cream (0.025%, Preferid®, Gist-Brocades, The Netherlands) a combination cream was obtained having the following composition:
[0053]
Amount (mg)Budesonide0.125Trisodium phosphonoformate hexahydrate15Sodium citrate0.6Citric adic0.3Sorbic acid0.3Cetostearyl alcohol30Paraffin liquid3Cetomacrogol 10006White soft paraffin15Arlatone (polyoxyethylene fatty acid ester)31Cetyl alcohol14Stearic acid14Mineral oil14Propylene glycol14Glycerol10.5Methyl p-hydroxybenzoate0.43Propyl p-hydroxybenzoate0.19Sodium hydroxide 2 M*Hydrochloric acid 2 M*Waterad 1000
example 4
Cream of Hydrocortisone 1%
[0056]
Amount (mg)Hydrocortisone10Methyl p-hydroxybenzoate2.0Propyl p-hydroxybenzoate0.5Glycerol0.03Ethanol5Isopropylmyristate50Amphisol10Paraffin30Paraffin, liquid40Macrogol stearate100Cetyl alcohol50Waterad 1000
[0057]This cream is commercially available as Hydrokortison kräm 1% ACO, Kabi Pharmacia AB, Sweden.
example 5
Cream of Foscarnet 2.4% and Hydrocortisone 1%
[0058]
Amount (% by weight)Trisodium phosphonoformate hexahydrate2.4Hydrocortisone1Phospholipids30Waterad 100
PUM
| Property | Measurement | Unit |
|---|---|---|
| healing time | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| swelling | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com