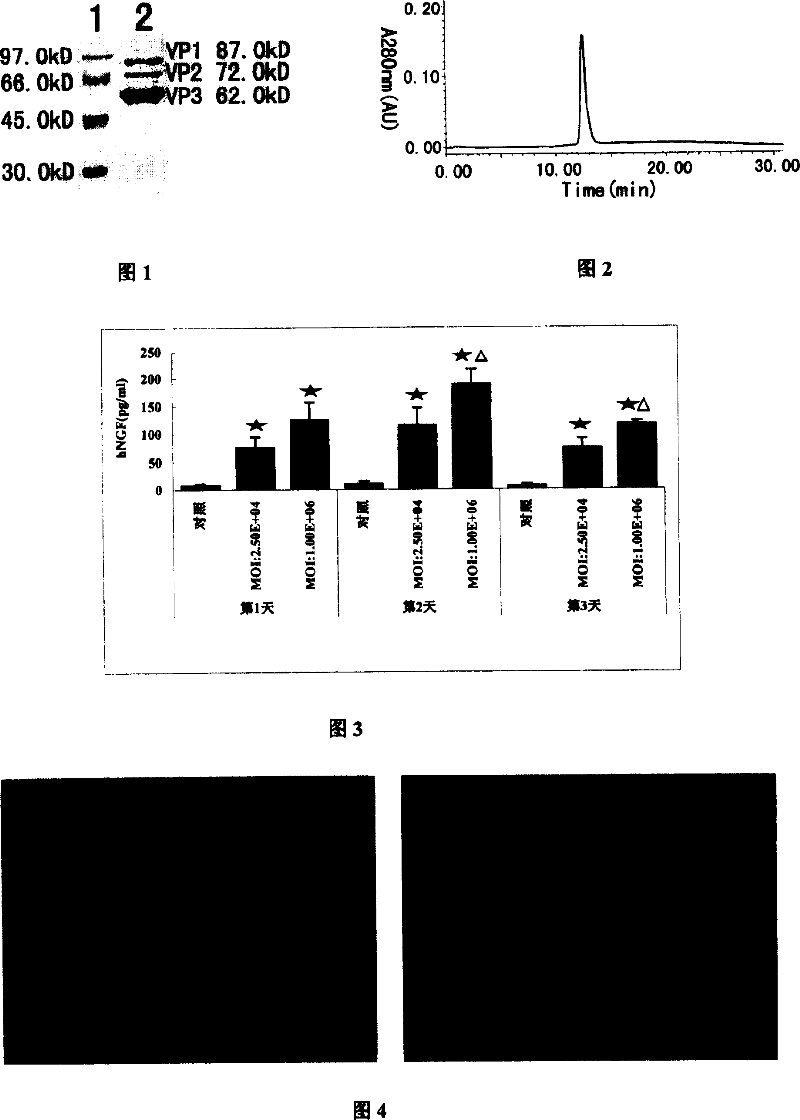
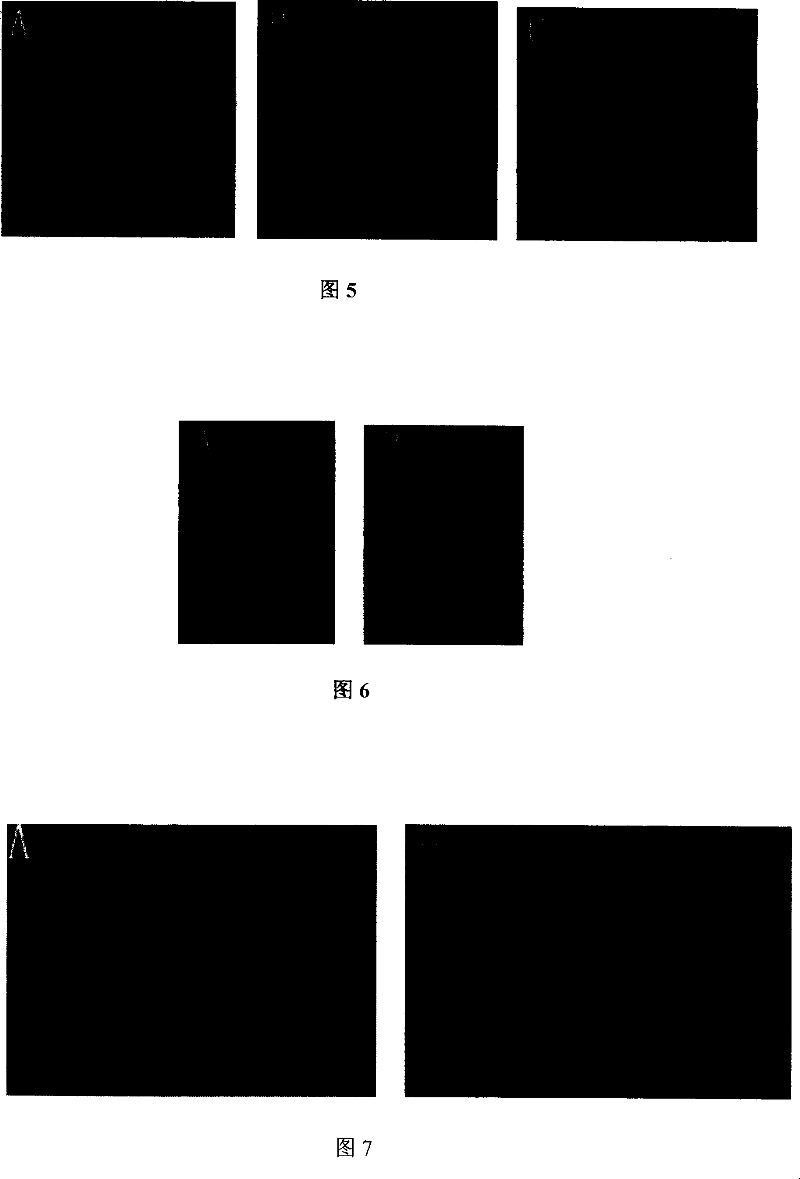
Adeno-associated virus vector preparation and medicinal composition containing it and its use
A virus carrier and composition technology, applied in the field of adeno-associated virus carrier preparations, can solve the problems of virus stability and infectivity decline, virus particle aggregation, precipitation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1 vector construction
[0066] Firstly, the total RNA of human liver tissue was extracted, and human hNGFβcDNA was amplified by RT-PCR (mRNA purification and RT-PCR kit were purchased from Promega, USA). -3', the length of the amplified fragment is 1067bp, and it is connected with the plasmid pUC18 to obtain the recombinant cloning plasmid pUC18 / hNGFβ. DNA sequence determination confirms that its nucleotide sequence is consistent with the literature records. The hNGFβ gene was ligated with rAAV-2 universal vector plasmid pSNAV-1 (WuZJ, Wu XB, HOU YD, Chin J viral 2000; 16:1-6.) to obtain the recombinant vector plasmid pSNAV-1 / hNGFβ, and transfected with the plasmid BHK-21 cells were screened under the pressure of G418 (neomycin) to obtain the vector cell line SN carrying the vector plasmid pSNAV-1 / hNGFβ. With the participation of the rep and cap genes), the virus was packaged and finally purified (Wu XB, Dong XY, Wu ZJ, et al., Chin Sci Bull 2000; 45:2071-20...
Embodiment 2
[0070] Example 2 In vitro expression of rAAV-2 / hNGFβ and determination of biological activity
[0071] 1. In vitro expression
[0072] The ability of rAAV-2 / hNGFβ to transduce cells was determined by measuring the content and biological activity of hNGFβ in the cell supernatant after infecting BHK-21 cells. With 1640 medium (RPMI 1640 medium purchased from American GIBCO Company) containing 10% calf serum (the calf serum is a product of HyClone Company), at 37 ° C, 5% CO 2 BHK-21 cells were cultured under these conditions. BHK-21 cells by 4×10 5 Each well was connected to a six-well plate, and after 8 hours, the MOI was 2.5×10 4 with 1.0×10 6 Add recombinant rAAV-2 / hNGFβ virions. Cells were washed twice with fresh serum-free 1640 before adding virus particles. And adjust the ratio of virus to cells (MOI) with serum-free 1640, then make up the final volume to 1ml with serum-free 1640, add to the 6-well plate cells that have been washed and prepared; and at 37 ° C, 5% CO ...
Embodiment 3
[0076] Example 3 Factors Affecting Mannitol-Induced BBB Opening
[0077] 1. The influence of mannitol concentration: the suitable concentration of mannitol is 22-28% (w / v)
[0078] Wistar rats were used as experimental animals, with a body weight of 250g±20g, and both ♀♂ were used. (n=20, 5 in each group) 4 groups of rats were treated at 37°C with 0.12ml·s -1 , 2 minutes after inputting 20%, 22%, 25%, and 28% mannitol at a speed of 30 seconds, the gray levels of the left blue-stained areas of the EB group were 181.5±1.41, 115.5±1.32, and 81.1±1.21, respectively. and 80.25±1.46, and the percentages (%) of blue-stained areas of the left brain were 32.42±1.12, 49.85±1.70, 92.10±1.50, and 94.31±1.32, respectively. However, rats in the 28% mannitol group had bleeding points, and 28% mannitol was more likely to precipitate at room temperature, indicating that at 37°C, 0.12ml·s -1 , 30s given the above different concentrations of mannitol, it can be seen that the opening of the BB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com